What Is Cesium Iodide?
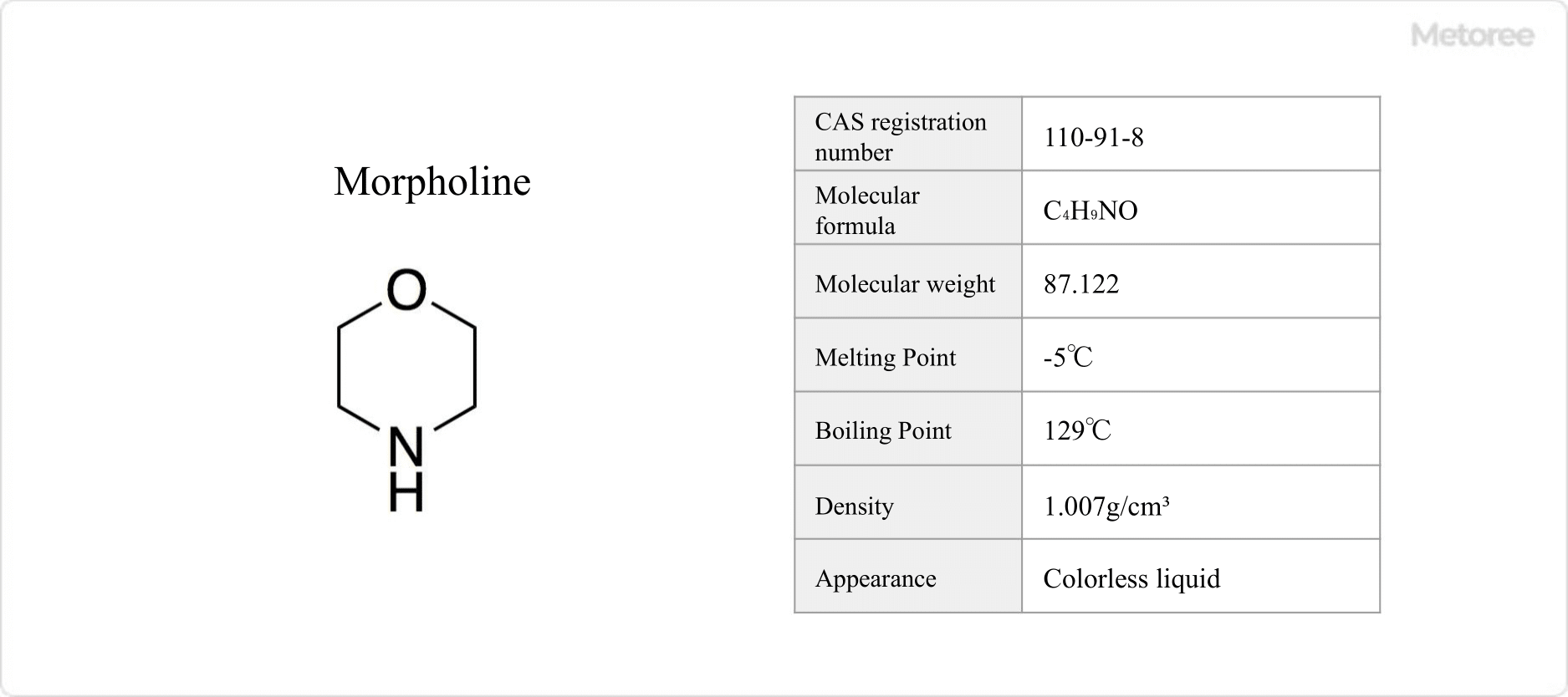
Cesium iodide is an odorless, white crystalline powder, recognized for its chemical formula CsI and molecular weight of 259.81. It is notable for its melting point of 621°C and boiling point of 1,280°C, demonstrating solubility in water and ethanol, limited solubility in methanol, and insolubility in acetone.
Uses of Cesium Iodide
Primarily utilized as a photocathode material in scintillators for detecting charged particles, cesium iodide is integral to scintillation detectors across particle physics and everyday applications. It also serves as a raw material for infrared transmissive glass, enhancing the efficiency of devices like nighttime surveillance cameras, infrared sensors, and night vision systems.
Properties of Cesium Iodide
This compound stands out for its white crystalline appearance, high melting point, and hardness. It boasts exceptional light transmittance across ultraviolet to infrared wavelengths and is highly effective in radiation absorption, making it ideal for photodetectors. Its high refractive index (1.79 at 589.3 nm) supports various optical applications, including lenses and optical fibers. Cesium iodide’s thermal stability upholds its structure at elevated temperatures, proving valuable for high-temperature instruments.
Structure of Cesium Iodide
CsI possesses a simple cubic lattice crystal structure, with cesium cations and iodide anions forming a dense lattice that significantly influences its refractive index and other properties.
Other Information on Cesium Iodide
How Cesium Iodide Is Produced
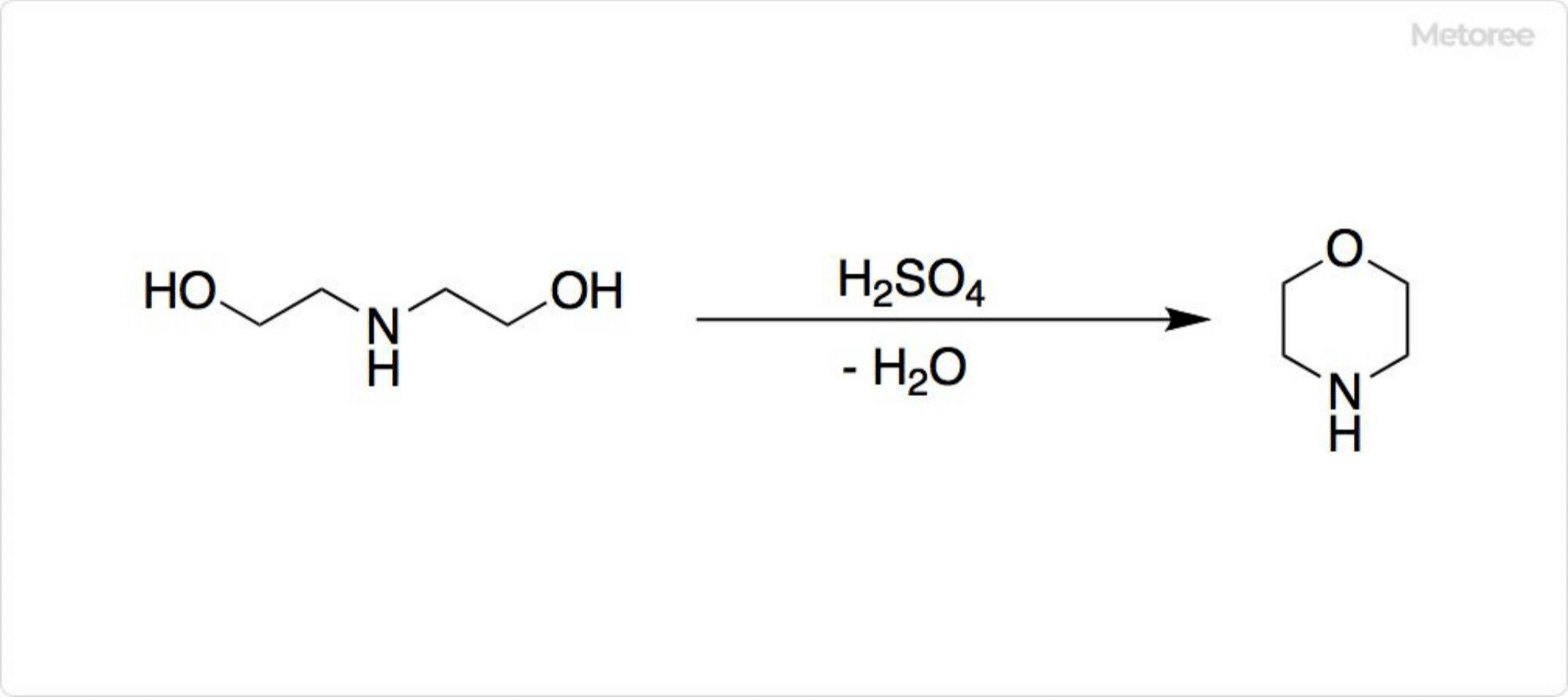
Industrial production methods include direct reaction, metathesis reaction, and solid-state reaction. While the direct reaction with metallic cesium and iodine yields high-purity CsI, it poses significant hazards. The metathesis reaction, involving cesium carbonate or hydroxide with an iodide salt, offers a safer, cost-effective alternative. The solid-phase reaction method is tailored for synthesizing CsI with specific crystal structures.