What Is a Flocculant?
A polymer flocculant is a polymeric agent that neutralizes the surface charge of particles suspended in water and causes them to flocculate.
It is mainly used to coagulate and suspend precipitate particles that cause pollution in wastewater.
A flocculant can be anionic, cationic, nonionic, or amphoteric, depending on the surface charge of the particles to be flocculated.
Uses of Flocculants
Flocculants are used in the following industrial applications: sludge concentration, volume reduction, and ore dressing.
- Anionic flocculants
Used in many industries that handle inorganic substances, such as metals, civil engineering, mining, ceramics, pulp and paper, chemicals, and foodstuffs. - Cationic flocculants
Often used in industries that handle organic materials such as human waste, sewage, paper pulp, and food products. - Nonionic flocculants
Used for acidic wastewater. - Amphoteric flocculants
Used when cationic flocculants are insufficient.
Flocculants are not used in wastewater treatment. If flocculant remains, it may cause organic contamination of ion exchange equipment or reverse osmosis equipment.
Principle of Flocculants
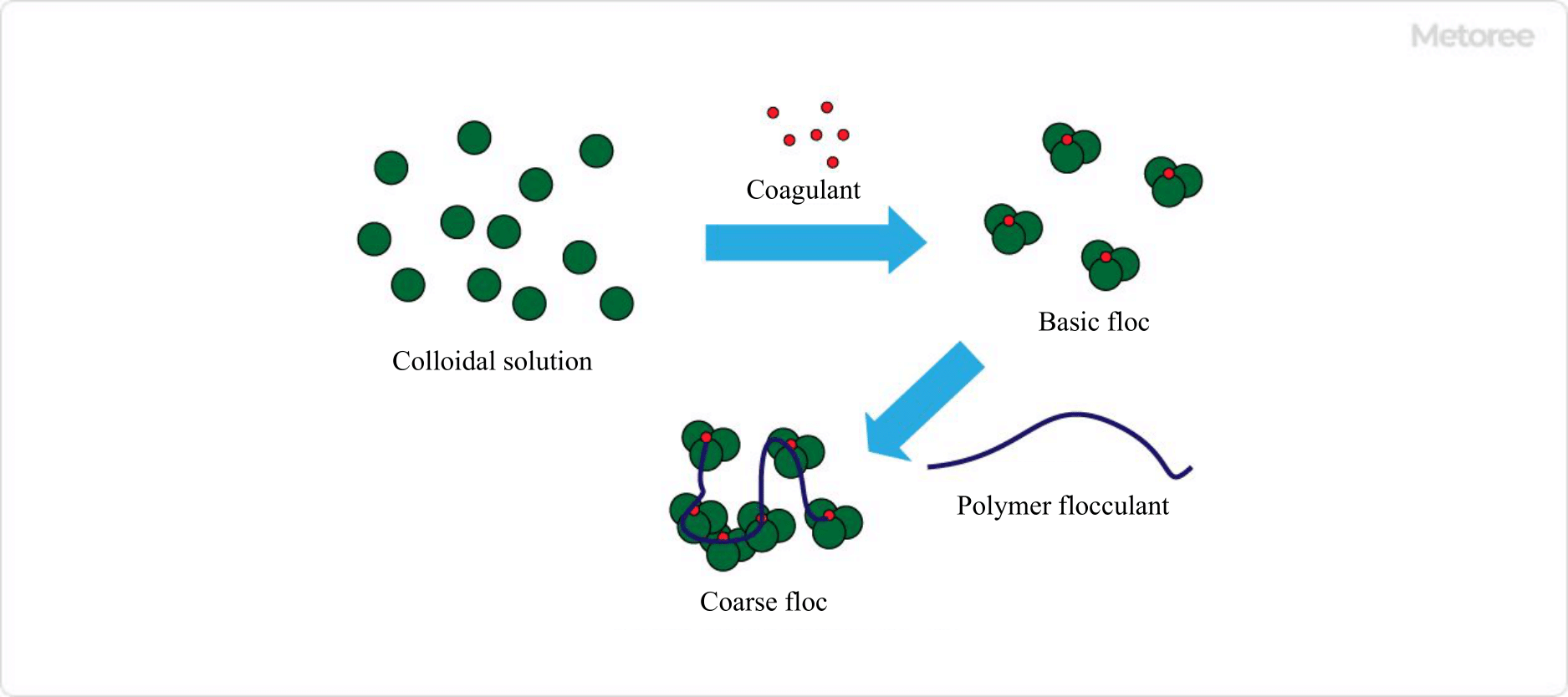
Figure 1. Image of flocculation by polymer flocculant
The mechanism by which flocculants cause flocculation and precipitation is as follows:
- Condensation
First, an inorganic coagulant (eg, poly aluminum chloride (PAC), iron sulfate, etc.), which has an opposite charge to the fine particles in the water, is introduced to neutralize the surface electrification of the suspended particles. The lumps formed at this time, that is, fine flocs, are called basal flocs. - Flocculation
Flocculants are added to the water in which the basic floc is generated. The active groups (polyacrylamide structure, etc.) of the flocculants interact with the small flocs, causing them to become entangled in the polymer, adsorbing and cross-linking, and growing into coarse flocs.
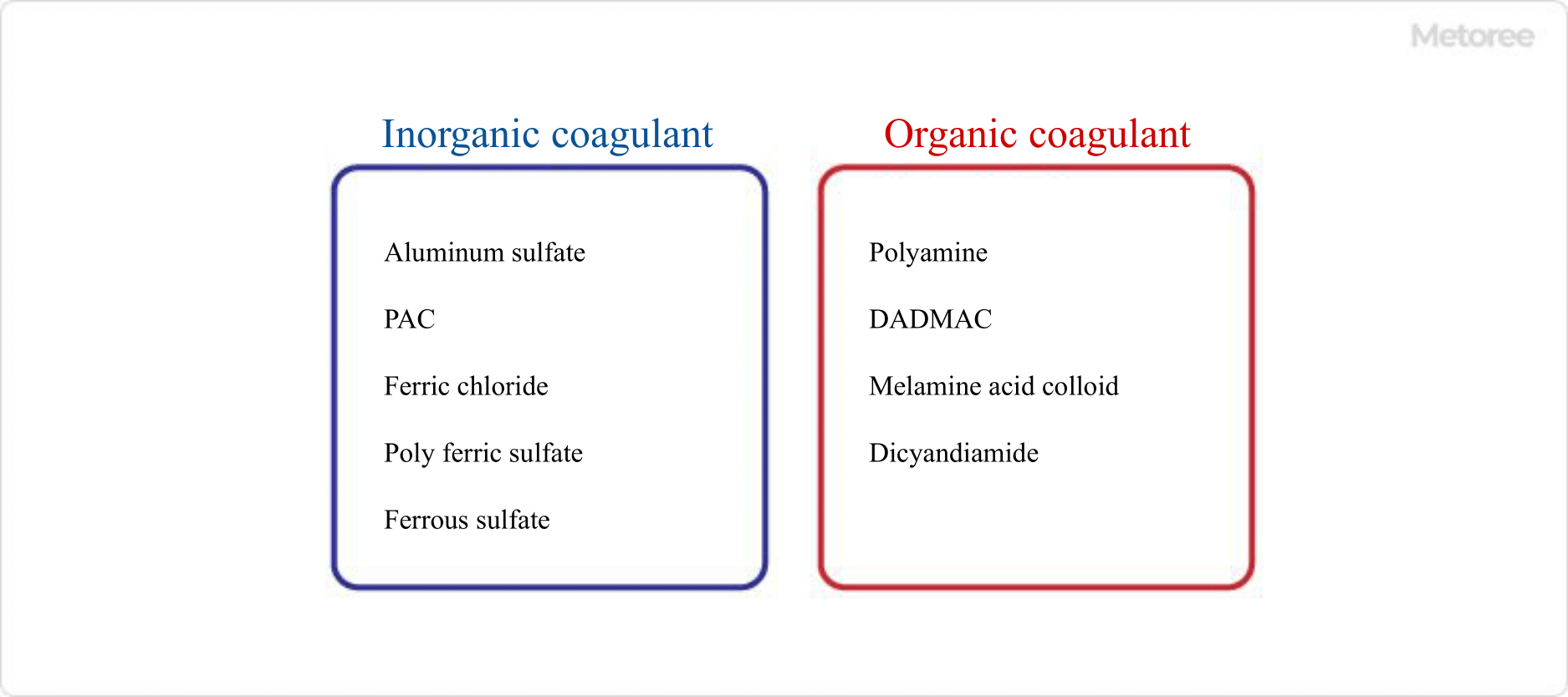
Figure 2. Major flocculants
Care must be taken in adjusting pH during sedimentation. This is because precipitation does not occur unless the pH environment is appropriate, and there is a risk of re-dissolution after precipitation. In addition, if more flocculants are added than the required amount, adsorption to the flocculants will occur first, resulting in insufficient cross-linking, making it difficult for flocculation to occur.
The appropriate pH for each flocculant is as follows
Anionic flocculants: pH 7 to 12
Cationic flocculants: pH 4-8
Nonionic flocculants: pH 4-8
Types of Flocculants
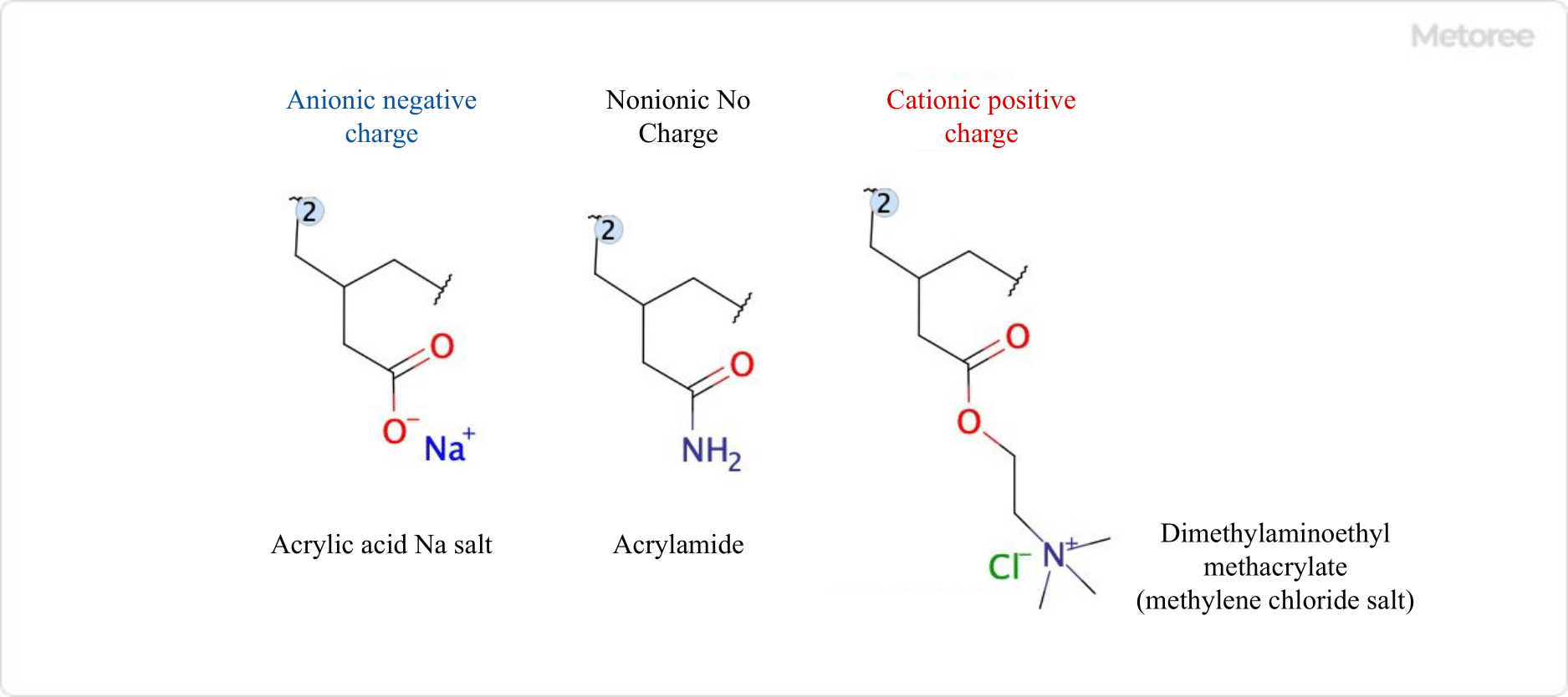
Figure 3. Monomer structures of major polymer flocculants
Flocculants can be classified in terms of ionicity and shape.
1. Ionicity
As mentioned above, flocculants can be anionic (anions), cationic (cations), nonionic (non-ions), or amphoteric, depending on the charge they carry. Anionic and nonionic types are mainly used to cross-link particles that have formed basic flocs with inorganic and organic coagulants and to turn them into coarse flocs. Cationic flocculants are often used to dewater biologically treated sludge.
2. Form
Flocculants are distributed in solid powder form and emulsion form. Powdered products are easy to handle for transportation and storage, but care must be taken when using them because of their low solubility. Usually, the powder is put into water in small quantities and stirred. If a large amount is added at once, only the surface will swell, and the undissolved particles may adhere to each other and form a large mass.
Dissolving itself also takes more time than emulsion-type products, and it is necessary to wait for a while while stirring. In addition, since the powder form absorbs moisture, it must not be stored in water. to use emulsion products if the flocculants tank is located outdoors.
Emulsion products are easier to dissolve than powder products and dissolve relatively quickly.
How to Select Flocculants
To select the best flocculants, it is necessary to consider various conditions. There are various factors such as the composition of the raw water to be added, pH fluctuation range, optimum amount to be added, temperature at the time of use, and the feeding point of the facility. In many cases, the type of additive is changed between summer and winter.
In the selection process, we conduct coagulation sedimentation tests (jar tester, cylinder tester, etc.) and dehydration desk tests (centrifugal dehydration test, belt press dehydration test, etc.) to select the appropriate brand and treatment conditions, followed by testing on actual equipment.