What Is Chromium Chloride?
Chromium chloride refers to a group of chemical compounds consisting of chromium and chlorine, with varying oxidation states: chromium(II) chloride, chromium(III) chloride, and chromium(IV) chloride.
Chromium(II) chloride is produced by reacting hot chromium metal with hydrogen chloride. Chromium(III) chloride forms when chromium metal is heated in a chlorine atmosphere. Chromium(IV) chloride, produced by heating chromium(III) chloride with chlorine at 600-700°C, is unstable and challenging to isolate.
Uses of Chromium Chloride
Chromium chloride catalyzes organic reactions, in chrome plating, and the manufacture of pigments, pharmaceuticals, and mordants. Specifically, chromium(II) chloride is essential in chrome plating technologies, enhancing corrosion resistance and surface hardness. In textiles, it helps bind dyes to fabrics. It is also used as a catalyst in olefin production and as a waterproofing agent.
Chromium(III) chloride plays a role in glucose metabolism, aiding in the activation of insulin-driven reactions, thus finding applications in pharmaceuticals for metabolic support.
Properties of Chromium Chloride
Chromium(II) chloride appears as colorless needle-like crystals, whereas chromium(III) chloride forms reddish-purple crystals. Chromium(IV) chloride exists as a gas at high temperatures.
Chromium(III) chloride, a Lewis acid, becomes more reactive in ligand substitution reactions upon the addition of zinc or a reducing agent like hydrochloric acid. The reduction to chromium(II) chloride facilitates immediate ligand replacement and electron transfer, enabling the regeneration of activated Cr(II).
Structure of Chromium Chloride
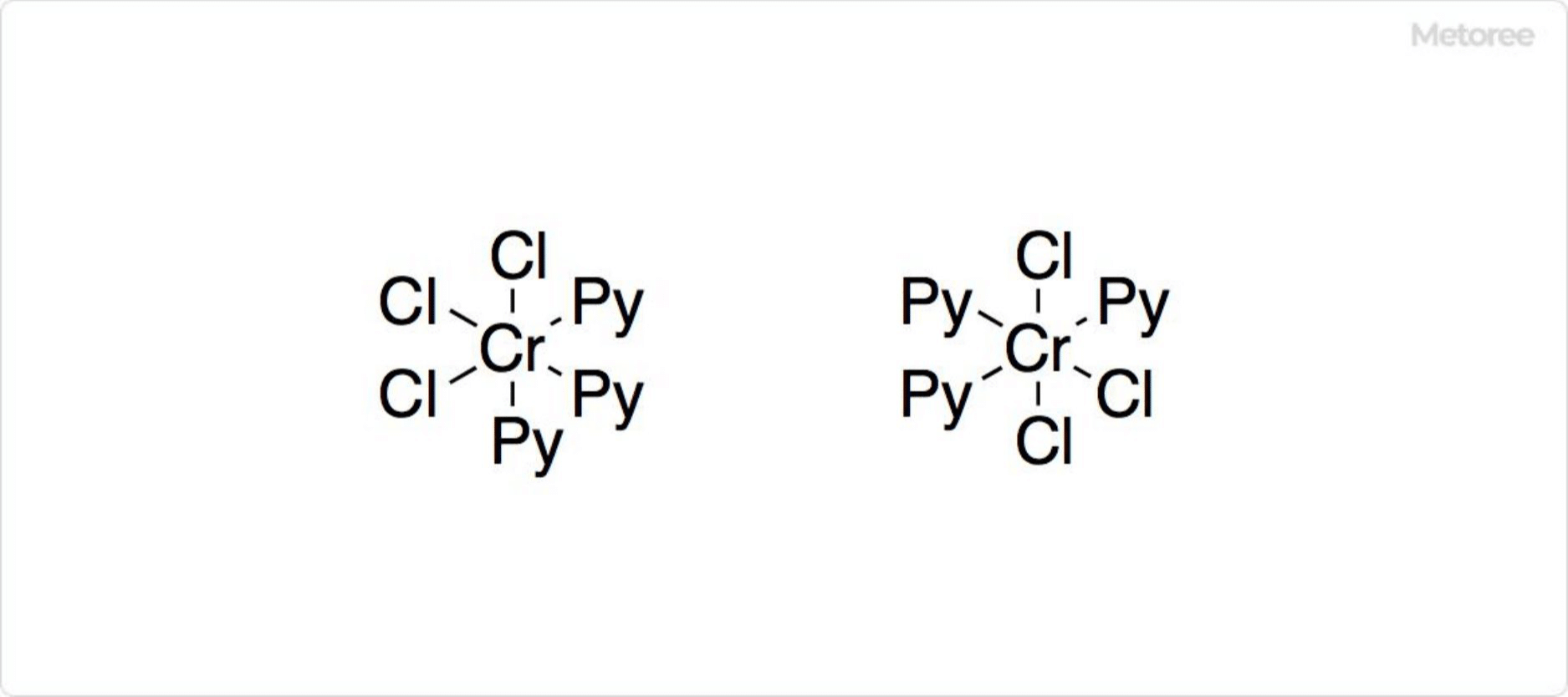
Figure 1. The complex of Chromium Chloride and Pyridine
Chromium chloride compounds are known by their oxidation states: Chromium(II) chloride (CrCl2), chromium(III) chloride (CrCl3), and chromium(IV) chloride (CrCl4). The molecular weights are 122.90 for CrCl2, 158.36 for CrCl3, and 193.81 for CrCl4. Anhydrous chromium(III) chloride is a purple crystal with limited water solubility.
Hydrated forms and pyridine complexes of chromium(III) chloride demonstrate its versatility in forming octahedral structures with coordination numbers of 6.
Other Information on Chromium Chloride
1. Synthesis of Chromium Chloride
Chromium(III) chloride anhydride is produced at high temperatures or by reacting dichromium trioxide with chlorine and carbon at 800°C. The hydrate form can be synthesized from hydrochloric acid and chromium, with dehydration possible using thionyl chloride. Chromium(II) chloride is obtainable by reducing chromium(III) chloride with hydrogen or reacting chromium acetate with hydrogen chloride.
2. Chromium Chloride as a Raw Material
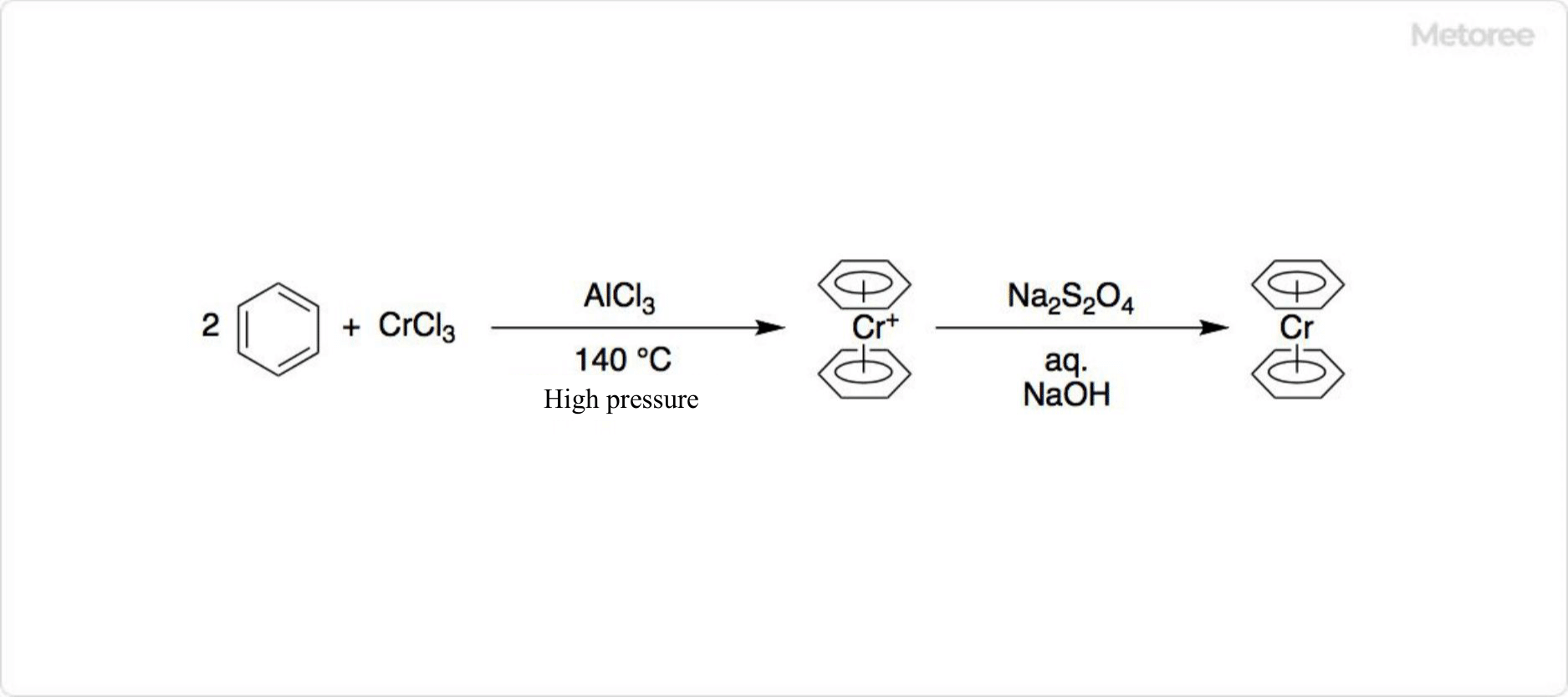
Figure 2. Synthesis of Organochromium Compounds
Anhydrous chromium(III) chloride is crucial in organometallic chemistry for synthesizing various organochromium compounds, including diphenylchromium.
3. Reactions Using Chromium Chloride
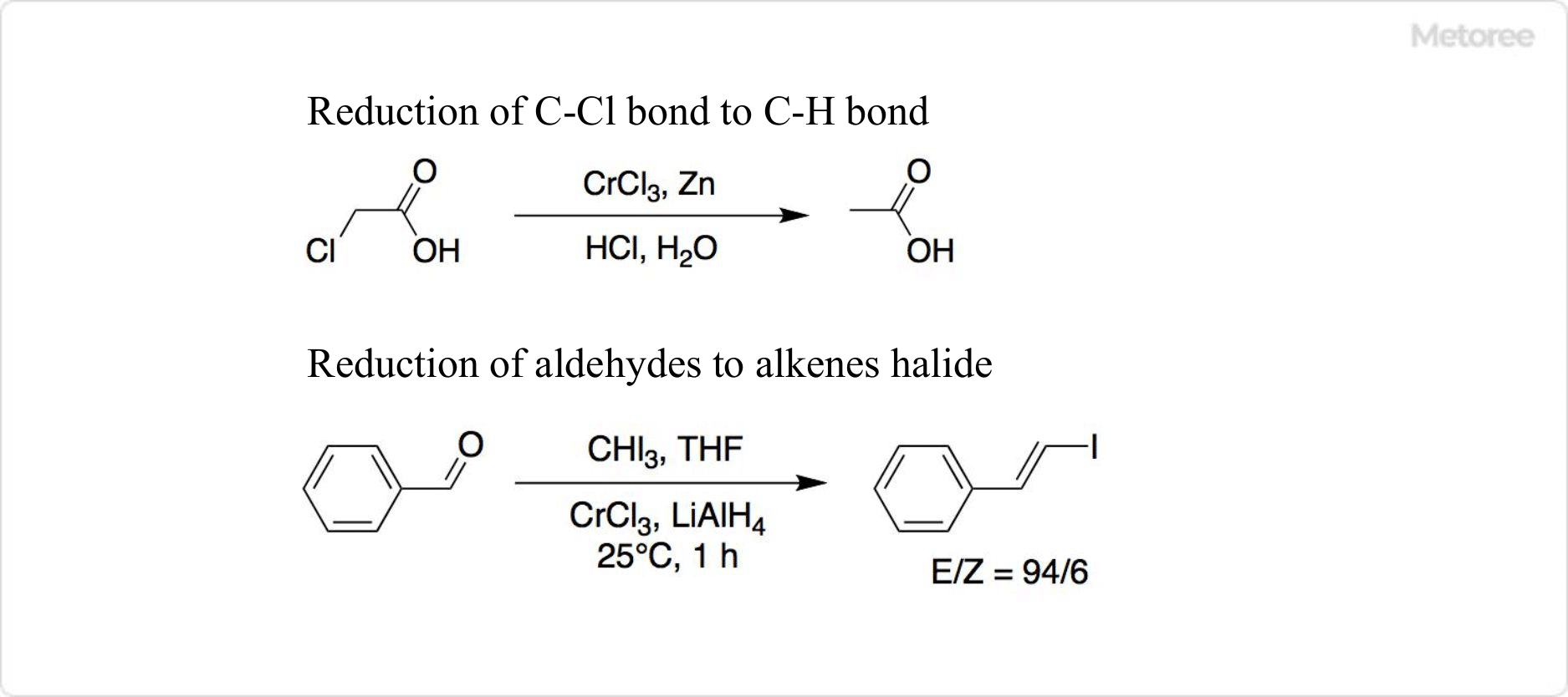
Figure 3. Reaction Using Chromium Chloride
Reduced from chromium(III) chloride, chromium(II) chloride acts as a reducing agent in organic synthesis, capable of converting C-Cl bonds to C-H bonds and reducing aldehydes to alkenes. A common reduction involves a 2:1 molar ratio of chromium(III) chloride to lithium aluminum hydride.