What Is an Electrometer?
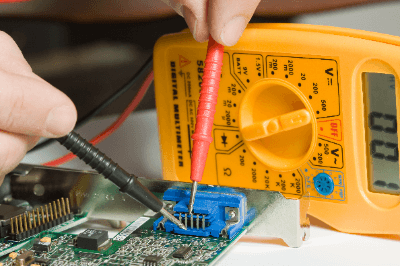
An electrometer is an instrument that can accurately measure electric potential or voltage by the potential difference between two points in a circuit.
By utilizing the electrostatic attraction and electrostatic repulsion between charged conductors, measurements can be made without current flowing from the circuit. Quadrant potentiometers and oscillating capacitance potentiometers are the two most common types of electrometers.
Quadrant potentiometers use a movable piece of metal (four separate disks suspended by a wire) to measure, in addition to potential, low currents, low power factor (an indicator of low effective utilization of power consumption in the power supply), and other parameters.
Oscillating capacitance electrometers convert a DC input into AC by changing the capacitance in parallel with the input, and then amplify it further to read the output. However, since it requires skill to handle, digital multimeters have been widely used in recent years.
Uses of Electrometers
Potentiometers are often used when evaluation with ordinary voltmeters is difficult. Specific applications include the following:
- When measuring electromotive force in power supplies with high internal resistance.
- When a current cannot be obtained even though the voltage is high, such as in the case of piezoelectricity in crystalline materials, friction electricity, etc.
- When current can be applied but polarization of the anode and cathode occurs, making accurate measurement of electromotive force impossible.
Compared to ordinary voltmeters, electrometers have a very high input resistance on the order of KΩ. Therefore, they are used to measure electric potential, insulation resistance, electric charge, and small currents in fields such as radiation, static electricity, and insulating materials.
Principle of Electrometers
In the case of surface potential meters, which measure static electricity, electrometers use the phenomenon of electrostatic induction to convert the induced charge from a charged object into a current and measure the charged potential from the AC voltage value.
The surface potential sensor, which is the measuring part of the surface potential measuring instrument, uses the electrostatic induction phenomenon. The electrostatic induction phenomenon refers to the phenomenon that when a charged object is brought close to a conductor, charges of the opposite polarity of the charged object are attracted to the side closer to the charged object.
In electrometers, when the sensing electrode receives the electrostatic field strength (a value indicating the strength of the electric field, which is proportional to the charged potential of the charged object), an induced charge is generated. When the electrostatic field strength is varied periodically at the vibrating electrode, the induced charge also changes periodically in tune, and a displacement current flows from the sensing electrode to ground (earth pole). By converting the current that flows in this case into an AC voltage signal with a resistor, it is possible to measure the charged potential of a charged object.
The measured value is affected by distance, object size, and environmental conditions (temperature, humidity, etc.), so comparisons must be made with these factors constant.
Other Information on Electrometers
1. About Human Body Potentiometers
Among electrometers, a human body potentiometer is an instrument used to accurately measure the potential of the human body. Using a human body potential meter, it is possible to determine, based on the measured potential values, whether or not electronic devices carried by the worker will be destroyed due to electrical charging or discharge current from tools.
The human body potential meter can also be used to evaluate the extent to which anti-static measures such as wristbands, electrostatic mats, and electrostatic shoes can reduce the potential of the human body.
2. Points to Note About Redox Electrometers
In the case of redox electrometers, care must be taken not to dry out the tip of the electrode because the potassium chloride in the electrode will precipitate and block the hole at the tip. To average the water, stir the water with the electrode while measuring.
If the oxidation-reduction electrometer readings show an abnormality, it is most likely due to water stains or oxide film adhesion. When water stain adheres to the sensor, lightly scrub the tip with a soft brush-like object and rinse it with tap water.
If the oxidation-reduction potential value still shows an abnormality, wash it with a soft brush or something similar with diluted neutral detergent. When oxide film adheres to the sensor, it is necessary to use a detection pole polisher.
Oxide film may form even if the sensor has just been purchased because of the generation of oxygen due to shaking during transportation. Also, when measuring clean water, care should be taken to avoid oxide film formation due to the high oxygen content.