What Is a Methanol Fuel Cell?
Methanol fuel cells are a type of fuel cell that directly uses methanol as fuel.
Fuel cells are fueled by hydrogen, but the introduction of fuel cells has been a hurdle due to the need for large-scale equipment to generate hydrogen gas and the high cost of equipment to transport and store explosive hydrogen gas.
However, methanol, being a liquid, can be stored in a smaller volume than hydrogen gas, making it possible to downsize fuel cells. It is also easier to handle than hydrogen gas, making the introduction of fuel cells easier. For these reasons, applications in mobile devices are expected.
Uses of Methanol Fuel Cells
Methanol fuel cells can be used just like regular batteries as long as the batteries can generate electricity.
Although the switch from gasoline-powered to fuel cell vehicles is underway to reduce carbon dioxide emissions, most current fuel cell vehicles are hydrogen-powered. This is because there are still some challenges in generating electricity with methanol fuel cells.
However, as methanol fuel cells become more common, they could be used to power cars and rechargeable batteries for smaller smartphones and other devices.
Principle of Methanol Fuel Cells
There are two main types of methanol fuel cells: direct and reforming.
1. Direct Methanol Fuel Cell
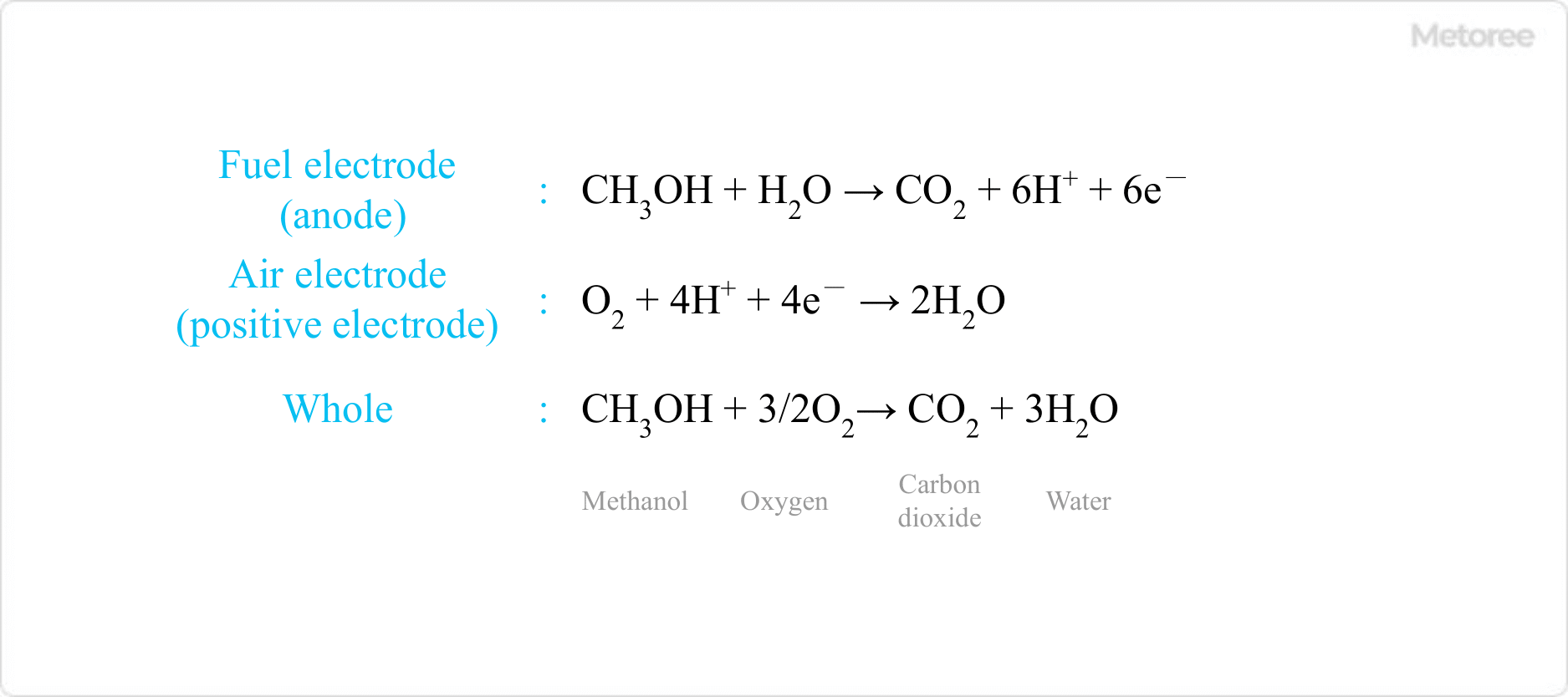
Figure 1. Reaction equation of a methanol fuel cell
Methanol fuel cells are fuel cells that use methanol directly as fuel, and are often referred to as methanol fuel cells. It consists of a fuel electrode (anode), where methanol reacts, and an air electrode (cathode), where oxygen from the air reacts. The reaction equation at each electrode is shown in Figure 1.
The reaction of methanol in aqueous methanol solution generates electrons through the action of a catalyst such as platinum in the fuel electrode, which continues to react as long as the fuel continues to be used. By generating electricity while supplying methanol, the fuel can be used for a long period.
2. Reforming Methanol Fuel Cell
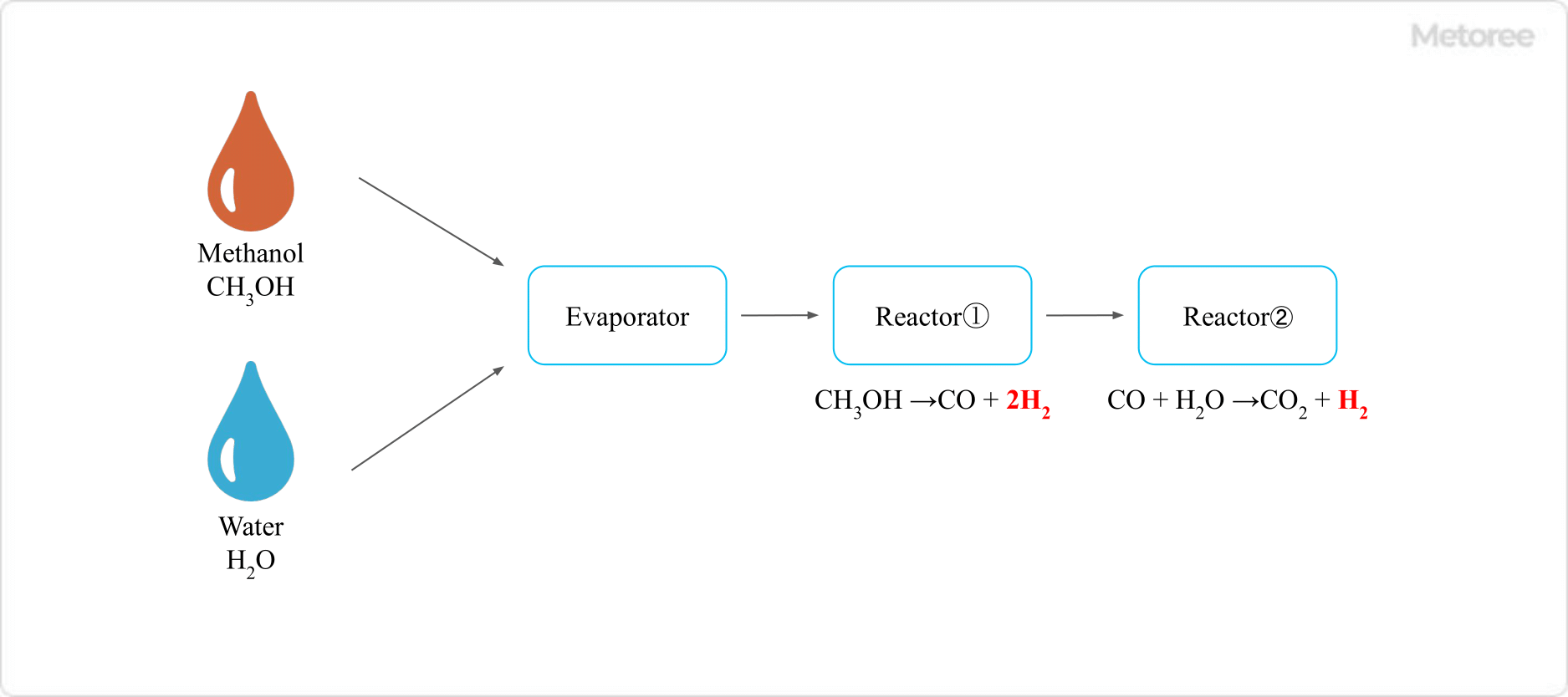
Figure 2. Modification of methanol
Reforming refers to extracting hydrogen from methanol. Since the extracted hydrogen is used as fuel to generate electricity, the principle of power generation is exactly the same as that of general hydrogen fuel cells, but since the starting material is methanol, these cells are also sometimes referred to as methanol fuel cells.
In methanol reforming, methanol is mixed with water vapor and reacts with a catalyst such as copper, zinc oxide, or aluminum oxide, which ultimately decomposes it into hydrogen and carbon dioxide.
Since this reaction is endothermic, heat must be supplied from an external source to advance the reaction. Although it is preferable to react at high temperatures to accelerate the progress of the reaction, the catalytic activity of the above catalysts is deactivated at temperatures above 300°C.
Other Information on Methanol Fuel Cells
1. Characteristics of Methanol Fuel Cells
Typical characteristics of methanol fuel cells include:
- Small size
- Small installation cost
- Quiet operation
- Low maintenance
While a typical fuel cell requires a hydrogen tank as well, methanol fuel cells can be made smaller because there is no need for a hydrogen tank. In addition, they do not rotate a turbine as in thermal or nuclear power generation, so they can generate electricity with quiet operation.
2. Challenges of Methanol Fuel Cells
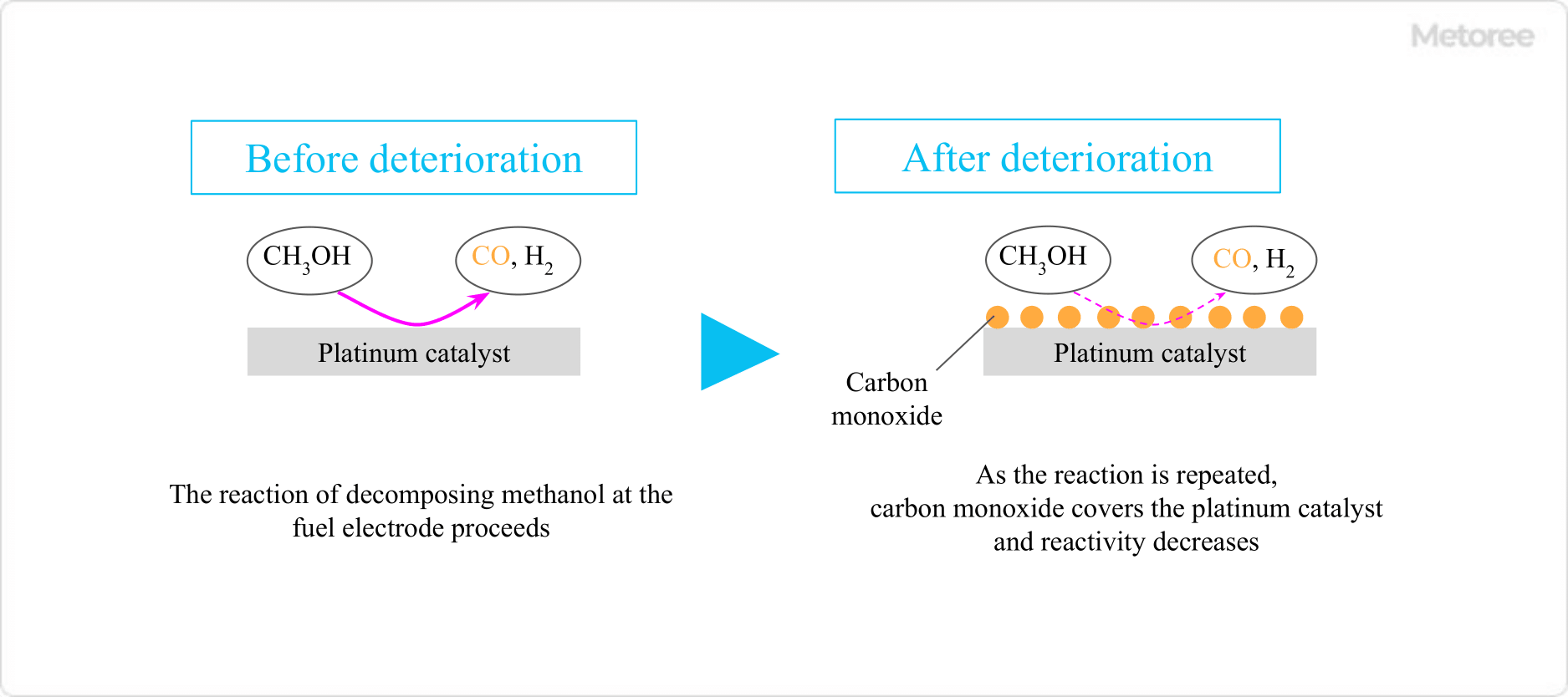
Figure 3. Decrease in reactivity of methanol fuel cell
In methanol fuel cells, the catalyst on the anode side that comes into contact with the methanol solution is contaminated by carbon monoxide, an intermediate product, resulting in reduced reactivity. This also shortens the life of the fuel cell itself.
Another major issue is the methanol crossover phenomenon, in which methanol permeates the electrolyte and reaches the air electrode, resulting in lower power generation efficiency and lower battery voltage. It can be said that fuel cells using methanol, which is inexpensive and easily stabilized but has a short product life, will take time to become widely used.