What Is Polyvinylidene Chloride?
Polyvinylidene chloride (PVDC) is a synthetic resin belonging to the amorphous thermoplastic family of polymers polymerized with chlorine-containing vinylidene groups.
The structure is represented by -[CH2CCl2]n-, and the CAS number is 9002-85-1. It is a colorless, transparent synthetic resin that is supplied to the market in film or fiber form.
Compared to other sheet resins, it has superior resistance to heat, acids, and alkalis, and has low oxygen and moisture permeability, making it suitable for use in food wraps. Copolymers with vinyl chloride (PVC) or acrylonitrile are used in products on the market, and they are rarely used alone.
Applications of Polyvinylidene Chloride
Polyvinylidene Chloride was originally developed primarily as a fiber. However, its most common use today is in household food wraps and to improve barrier properties from oxygen and humidity.
As a fiber, it is used today in various nets, carrier membranes for wastewater treatment, filtration cloth, and even doll’s hair.
At one time, dioxin emitted during combustion became a social problem, and attempts were made to collect it separately or replace it with other components. Today, however, with the improvement of incinerator combustion temperatures, it can be handled as ordinary combustible waste.
Principle of Polyvinylidene Chloride
The principle of Polyvinylidene Chloride is explained in terms of its synthesis method and properties.
1. Synthesis of Polyvinylidene Chloride
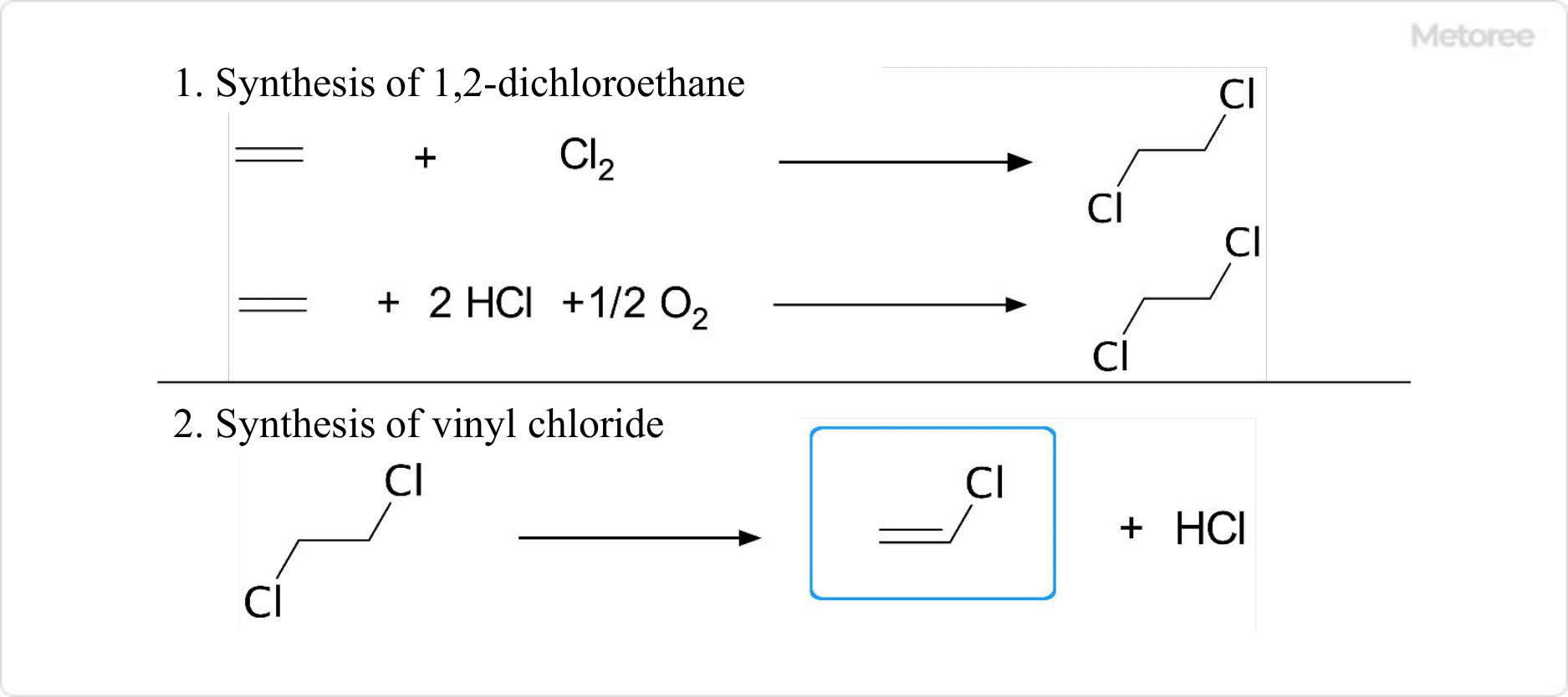
Figure 2. Synthesis of Poly (vinylidene chloride) – 1
The industrial manufacturing process for Polyvinylidene Chloride is as follows:
- 1,2-Dichloroethane is produced by reacting ethylene with chlorine or hydrogen chloride obtained by electrolysis of sodium chloride.
- Vinyl chloride is synthesized from 1,2-dichloroethane.
- After reacting vinyl chloride with chlorine to obtain 1,1,2-trichloroethane, calcium hydroxide or sodium hydroxide is reacted to synthesize vinylidene chloride monomer through a dichlorination reaction.
- Polyvinylidene Chloride Chloride can be obtained by polymerization with the addition of an emulsifier and a small amount of additives (such as vinyl chloride).
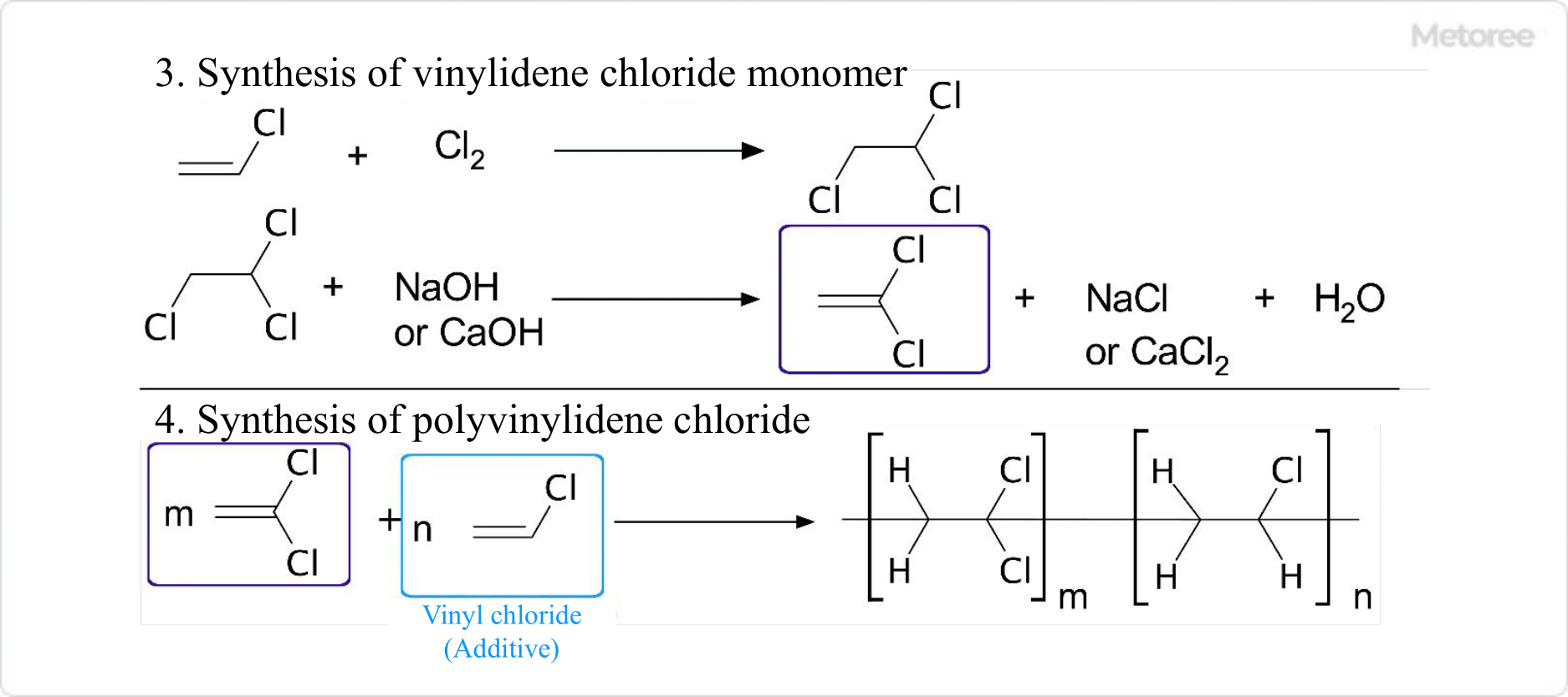
Figure 3. Synthesis of polyvinylidene chloride – 2
2. Properties of Polyvinylidene Chloride
Polyvinylidene Chloride is known to decompose at temperatures above 125 °C, producing hydrogen chloride.
Its moderate elasticity and its ability to provide both moisture-proofing and gas barrier properties are characteristics not found in other plastic films.
3. Process of Commercializing Polyvinylidene Chloride
When Polyvinylidene Chloride is commercialized as a film, which is its main application, it is formed by heating, melting, cooling, and stretching. At this point, a plasticizer such as tributyl acetyl citrate is added to make the material softener. The plasticizer remains in the product and a certain amount is eluted, but it is harmless to the human body.
To enhance the heat resistance and strength of Polyvinylidene Chloride, it is sometimes sandwiched between nylon or polypropylene in the manufacturing process to create a multilayer film. This method is used not only for film products but also for manufacturing latex and fishing nets.
Types of Polyvinylidene Chloride
Today’s main Polyvinylidene Chloride products include films and synthetic fibers. Film products are mainly used in household wraps and commercial food packaging materials. Synthetic fibers are often processed into products such as dust filters, filter cloths, nets for fishing, fishing lines, and blinds.
They are also sold as chemical materials intended for industrial applications, etc., and are available in powder, film, and other types. Film products are available in thicknesses of 0.0125mm, 0.033mm, 0.043mm, and in coil or square shapes.
For industrial use, the products are also supplied as waterborne high-barrier resin dispersions for coating textiles, paper, and other materials.