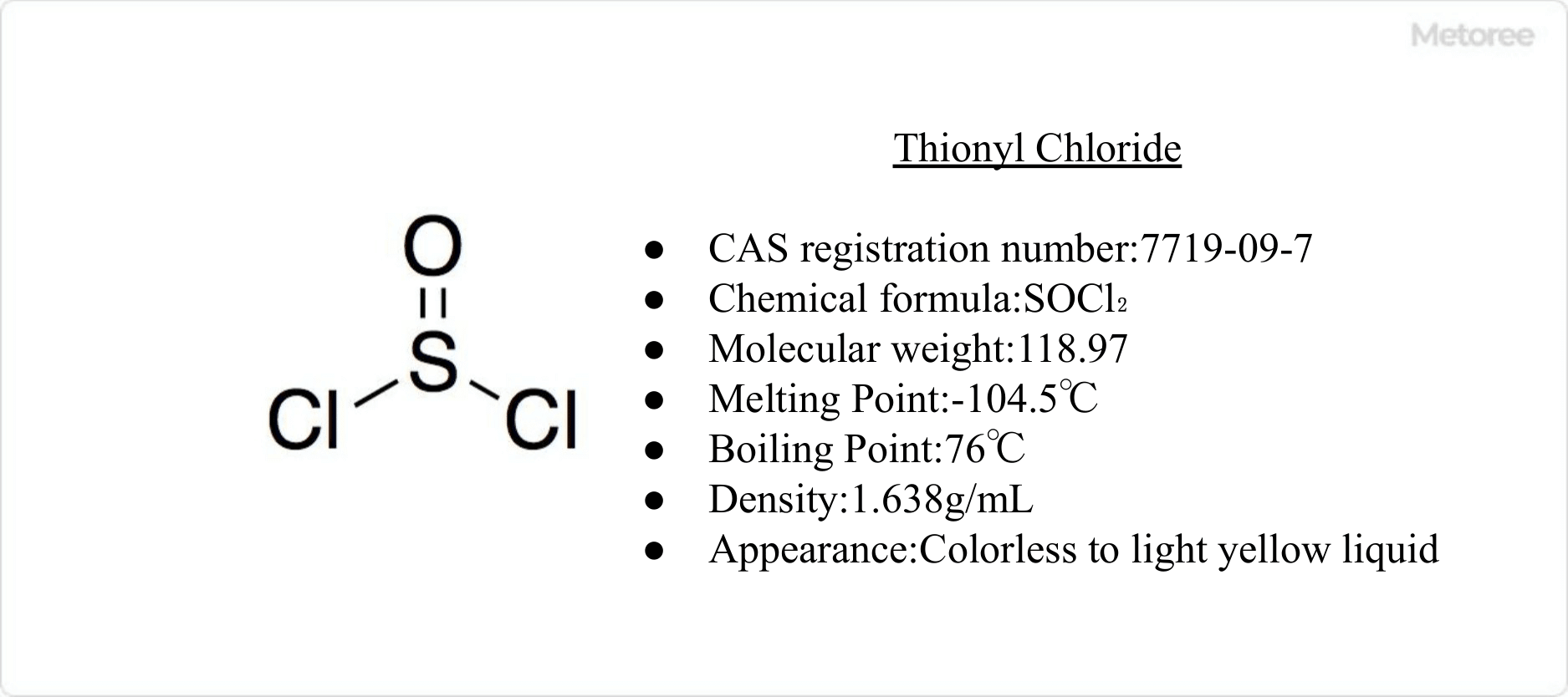
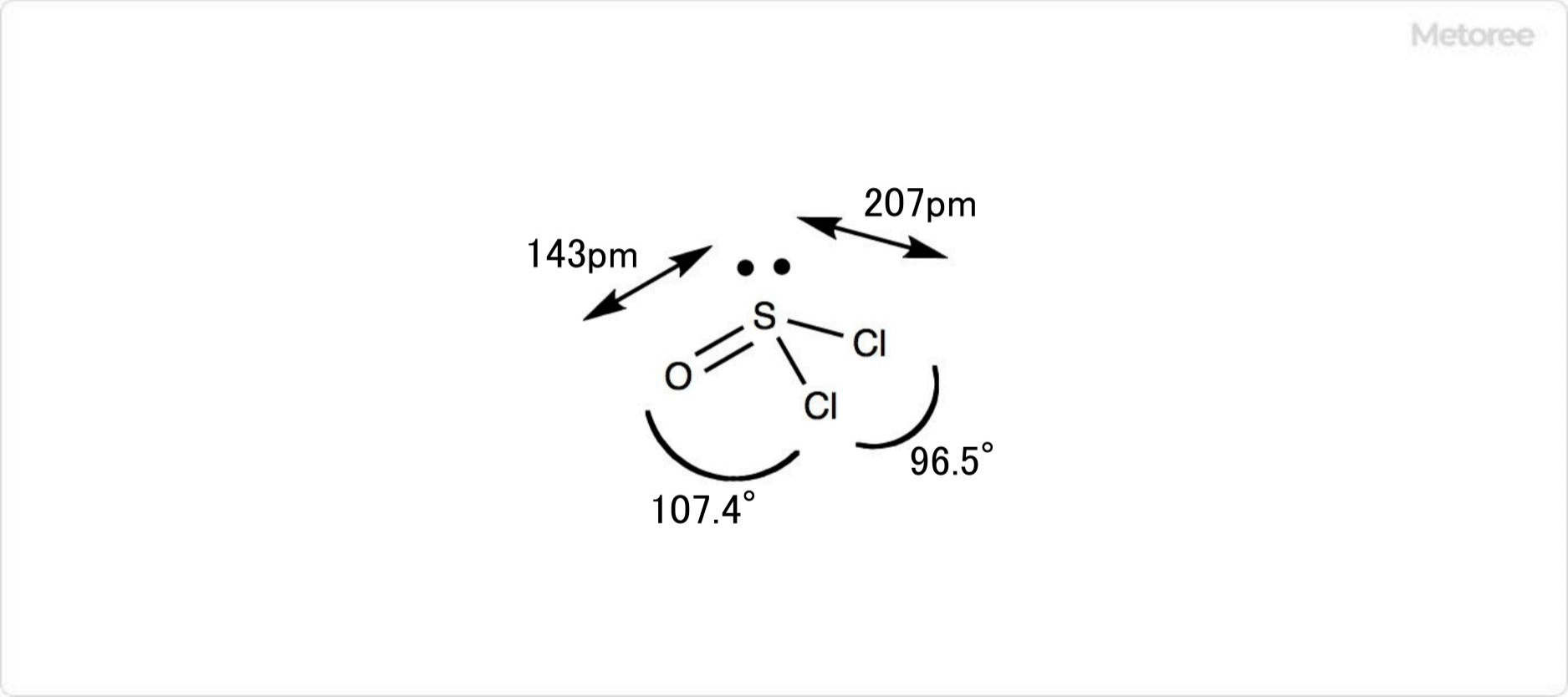
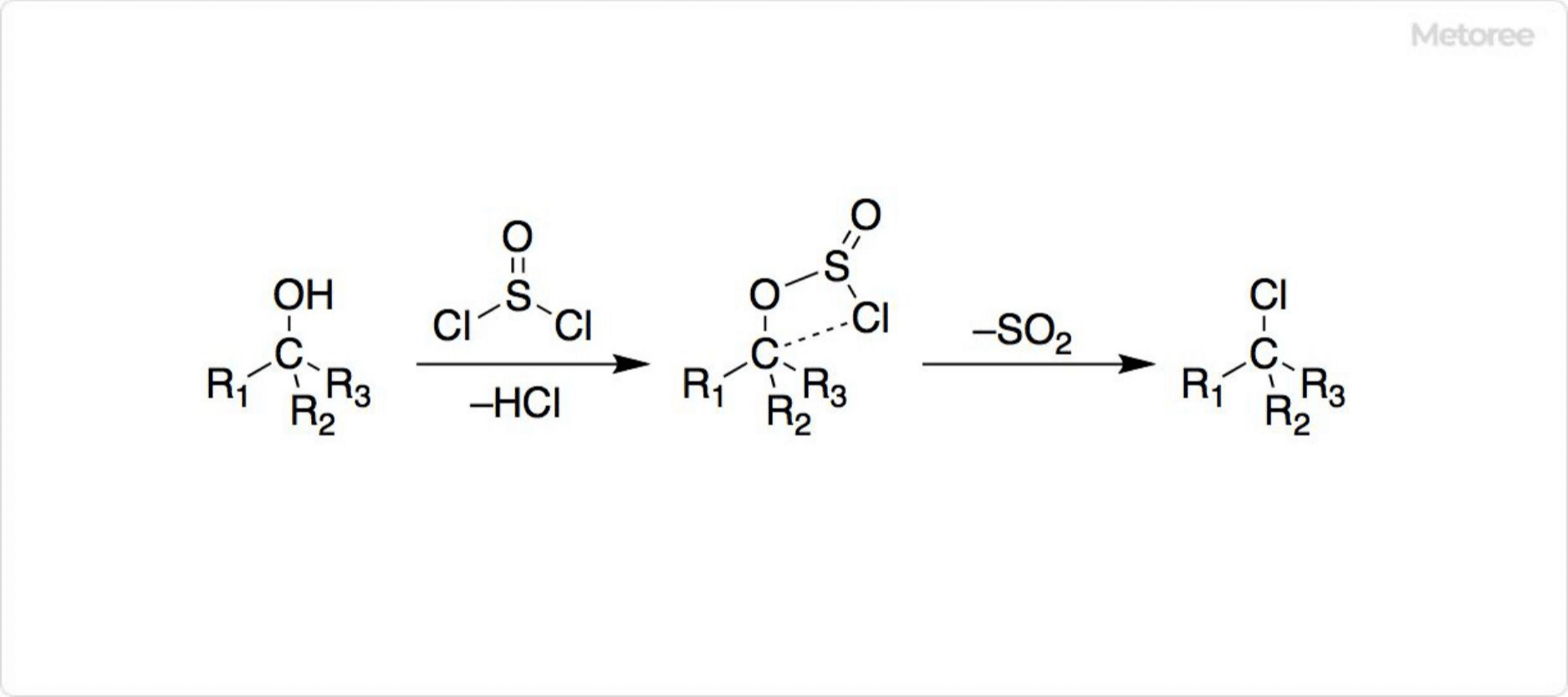
What Is Nickel Chloride?
Nickel chloride, a chemical compound, is known for its hazardous properties.
Classified by the GHS as acutely toxic, skin and respiratory sensitizer, carcinogenic, and toxic for reproduction, it also poses risks of specific target organ toxicity from single and repeated exposure. Nickel chloride exists in anhydrous, monohydrate, and hexahydrate forms.
As a carcinogen, it is regulated under various laws, which require it to be labeled and notified. It is also designated as a “priority evaluation chemical substance” and a “specified Class I designated chemical substance” under environmental protection laws.
Uses of Nickel Chloride
Nickel chloride is utilized in various industrial processes, such as in ammonia gas absorbents, nickel plating, dyes, and batteries. In complex chemistry, it serves as a precursor for nickel complexes and is used as an additive and reagent in organic synthesis, especially in nickel plating to increase solution solubility and as a source of nickel ions in plating baths.
Its role extends to providing chloride ions, enhancing the corrosive action necessary for nickel dissolution during anodic reactions.
Properties of Nickel Chloride
This compound appears as a pale yellowish-orange mass, crystal, or powder and is hygroscopic. The hexahydrate form, commonly used, is green to yellowish-green and deliquescent, soluble in water and alcohols.
Divalent nickel in nickel chloride has two unpaired electrons, leading to antimagnetic properties in planar four-coordinated nickel complexes.
Structure of Nickel Chloride
Nickel chloride, with the chemical formula NiCl2, has an anhydrous salt formula weight of 129.59 and a specific gravity of 3.55. The hexahydrate has a formula weight of 237.69 and a specific gravity of 1.92, sharing a crystal structure similar to cadmium chloride. The Ni-Cl bonds exhibit ionic characteristics, with each Ni2+ center coordinated to six Cl− ions.
In hexahydrate form, NiCl2•6H2O, only four water molecules directly bind to nickel, forming a complex with two additional water molecules weakly associated.
Other Information on Nickel Chloride
1. Nickel Chloride Synthesis Methods
Production involves dissolving metallic nickel, nickel oxide, or nickel carbonate in hydrochloric acid, followed by dehydration under hydrogen chloride gas to obtain the anhydrous form, indicated by a color change from green to yellow.
2. Reaction Using Nickel Chloride
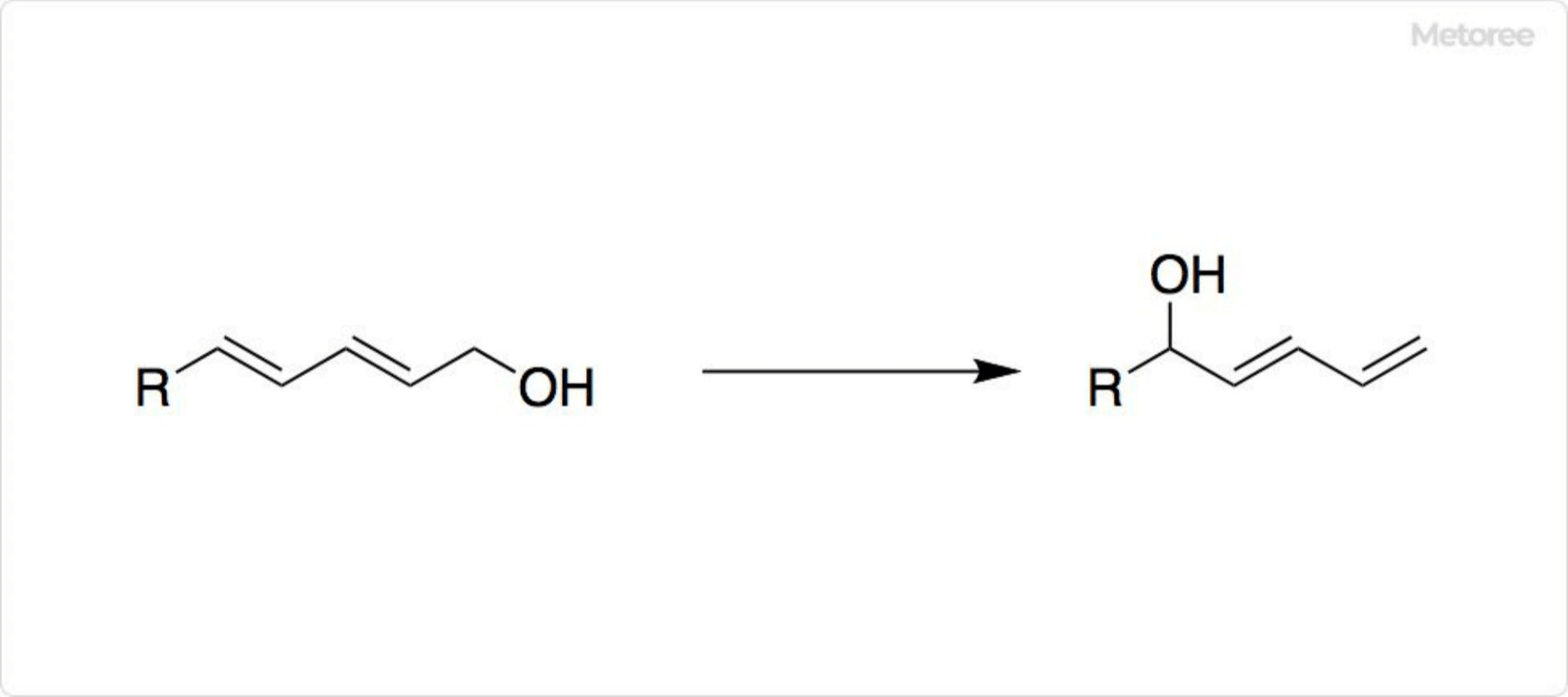
Figure 1. Reaction Using Nickel Chloride
Nickel chloride and its hydrates facilitate various organic synthetic reactions. It acts as a weak Lewis acid in regioisomerization of dienols and, combined with chromium(II) chloride, synthesizes allyl alcohols from vinyl iodide and aldehydes. It is also useful in reductions with lithium aluminum hydride and preparing nickel boride with sodium borohydride.
3. Complexes of Nickel Chloride
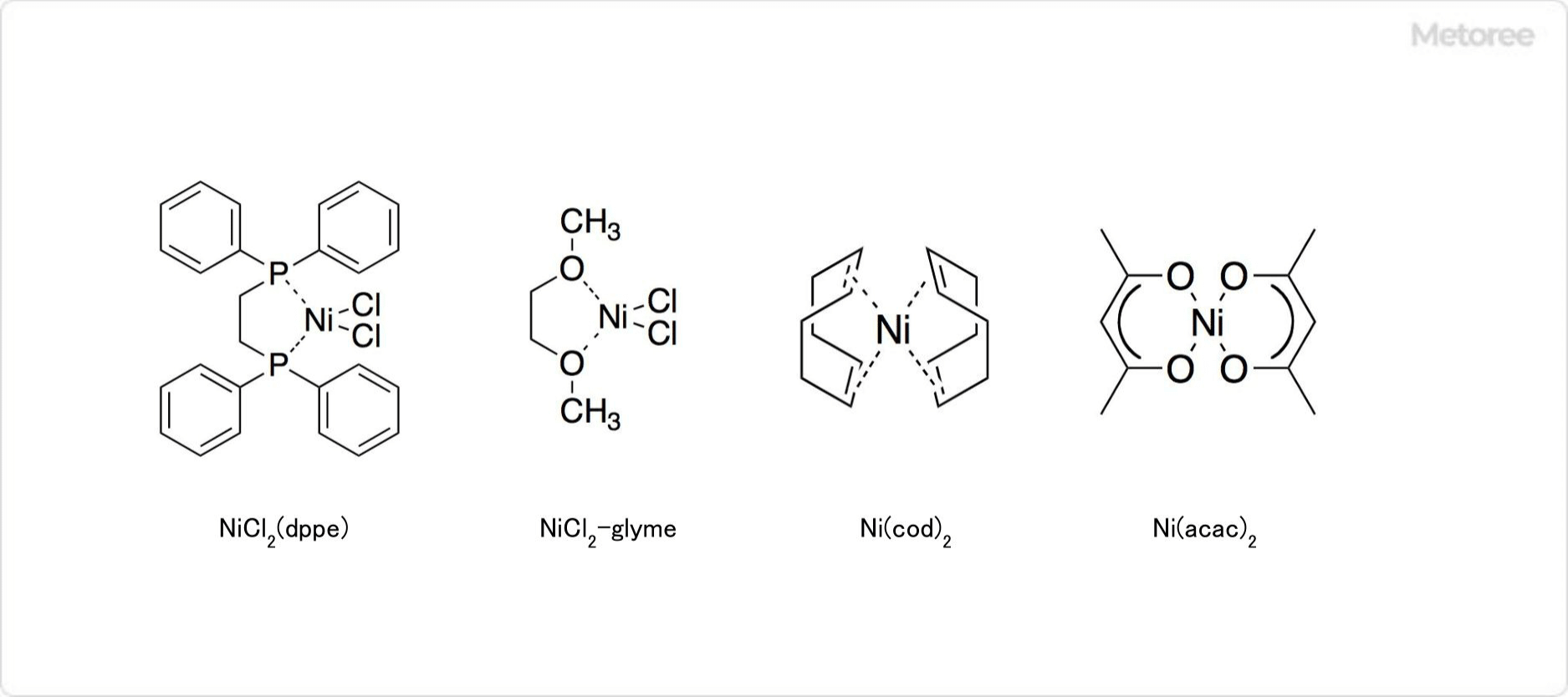
Figure 2. Complexes Obtained From Nickel Chloride
Nickel chloride hexahydrate readily exchanges its H2O molecules with ammonia, amines, phosphines, thioethers, and thiolates, forming various complexes such as the purple octahedral [Ni(NH3)6]Cl2, the orange planar tetrahedral NiCl2(Ph2PCH2CH2PPh2), the colorless planar tetrahedral [Ni(CN)4]2-, and the blue tetrahedral [NiCl4]2-.
4. Reactions Using Nickel Chloride Complexes
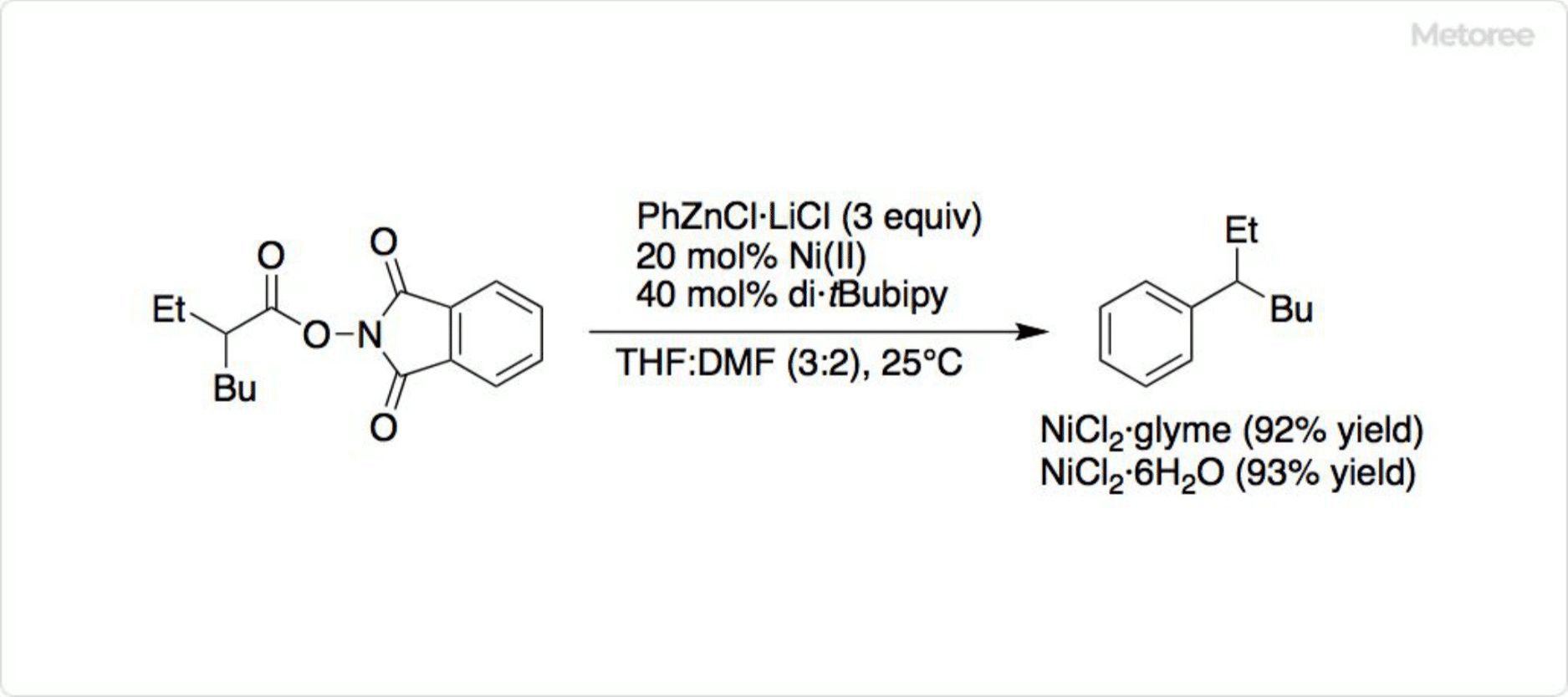
Figure 3. Reaction Using Nickel Chloride Complex
NiCl2-glyme complexes, more soluble than the hexahydrate, find use in various reactions. Nickel(II) acetylacetonate, a precursor to bis(1,5-cyclooctadiene)nickel, can be synthesized from nickel chloride.