What Is Thionyl Chloride?
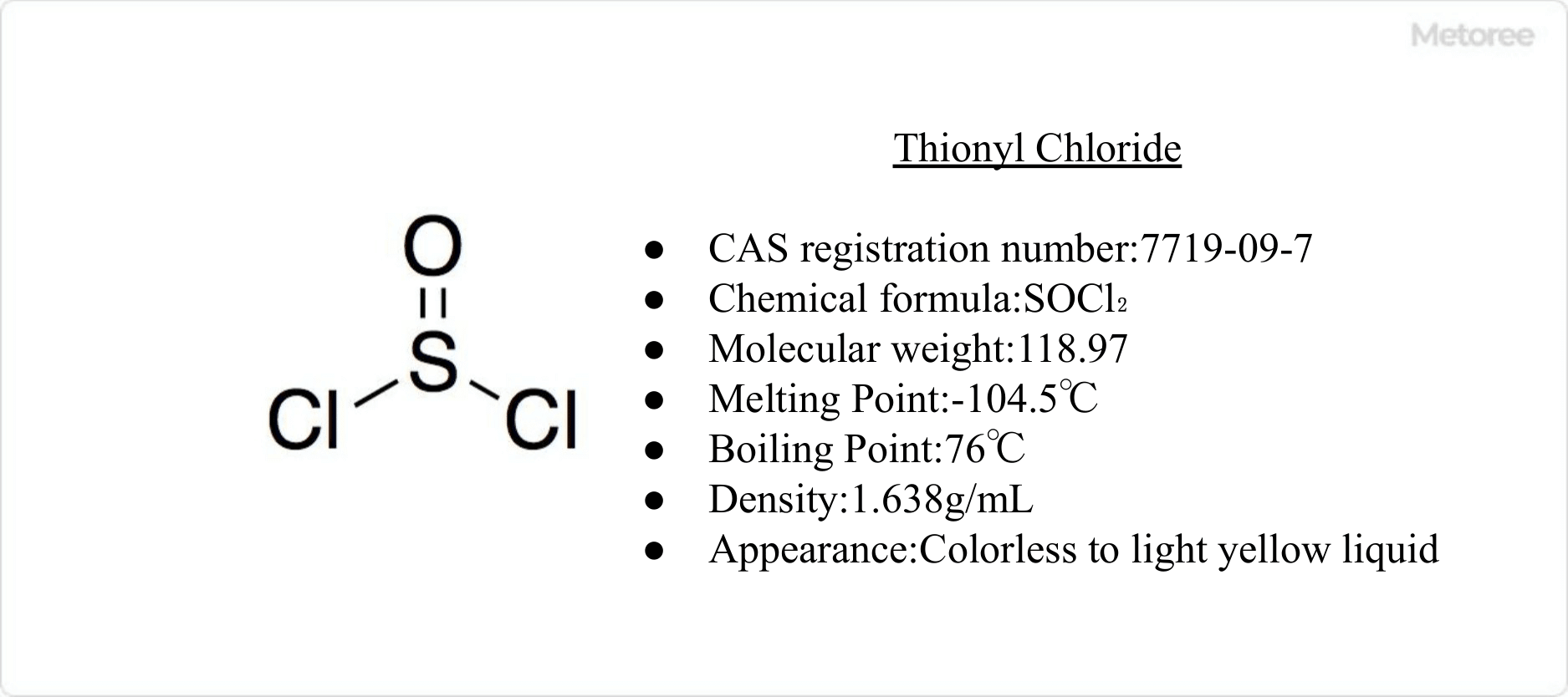
Figure 1. Basic Information on Thionyl Chloride
Thionyl chloride is a colorless liquid with a pungent odor and fuming properties.
It is also known as sulfinyl chloride or thionyl chloride. Thionyl chloride, in liquid or vapor form, is toxic because it is harmful to skin and mucous membranes.
Thionyl chloride is classified as a “deleterious substance” under the Poisonous and Deleterious Substances Control Law, and as a “hazardous and noxious substance” and “hazardous and noxious substance requiring notification of name, etc.” under the Industrial Safety and Health Law.
Uses of Thionyl Chloride
Thionyl chloride is used to replace hydroxyl and mercapto groups with chlorine atoms in the organic synthesis of dyes, medicine, and agricultural chemicals. Thionyl chloride can also be used to introduce sulfone groups by reacting with Grignard reagents. Other uses include use as a dehydrating agent to obtain anhydrous metal halides.
Thionyl chloride in liquid form is useful as a cathode active material in thionyl chloride lithium batteries. Thionyl lithium chloride batteries are widely used for backup of memory ICs, power supplies for electronic equipment, and power supplies for electricity, gas, and water meters.
Properties of Thionyl Chloride
Thionyl chloride has a fuming and pungent odor, a melting point of -104.5°C, and a boiling point of 76°C. It begins to decompose when heated above 140°C and decomposes completely only at 500°C. Decomposition of thionyl chloride yields sulfur dioxide (SO2), disulfur dichloride (S2Cl2), and chlorine (Cl2).
Thionyl chloride mixes with solvents such as benzene and chloroform. It also reacts with water in a vigorous exothermic reaction to form hydrogen chloride (HCl) and sulfur dioxide (SO2).
Structure of Thionyl Chloride
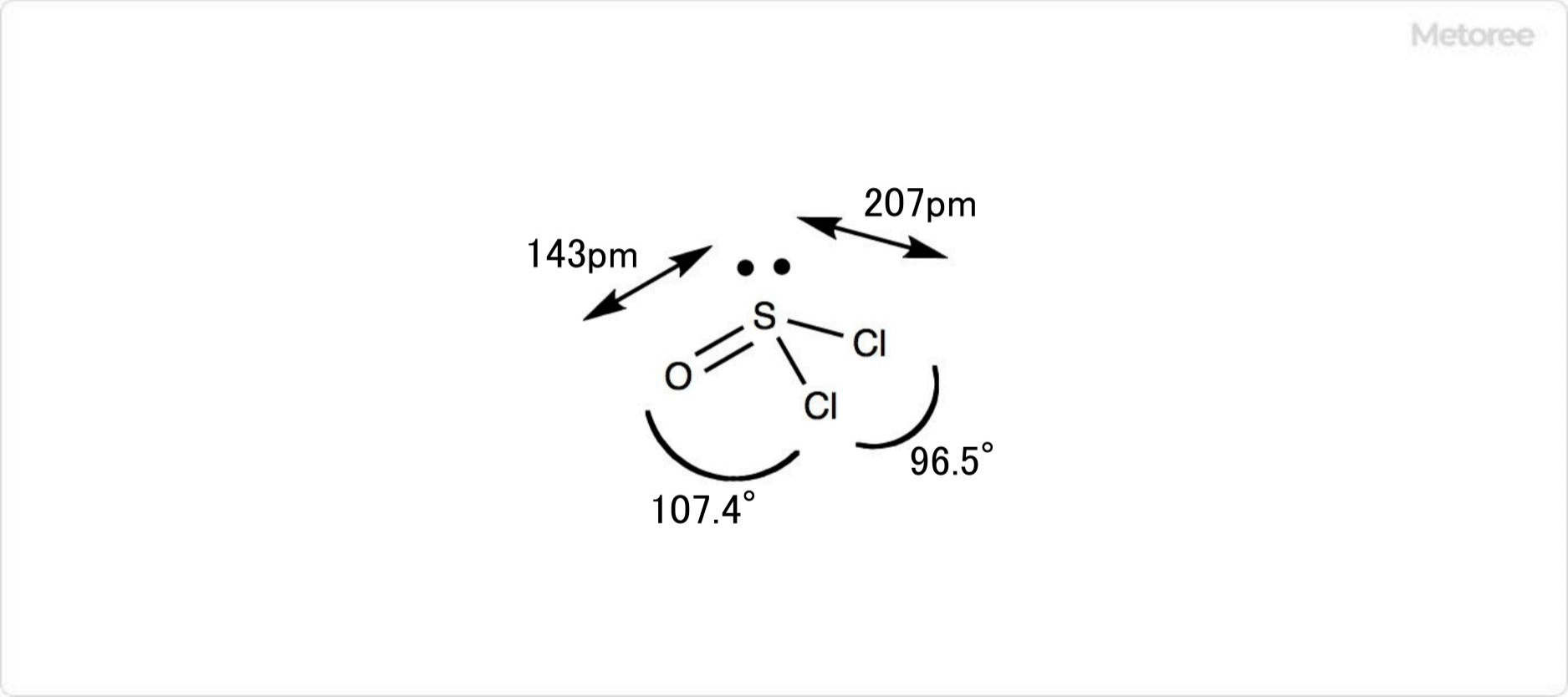
Figure 2. Detailed Structure of Thionyl Chloride
Thionyl chloride is a sulfurous acid chloride-like compound with the chemical formula SOCl2, a liquid with a molecular weight of 118.97 and a specific gravity of 1.65 g/cm3. The sulfur-oxygen bond (S-O) is approximately 143 pm and the sulfur-chlorine bond (S-Cl) is approximately 207 pm in length.
The thionyl chloride molecule has a triangular pyramidal shape. ∠O-S-Cl and ∠Cl-S-Cl are 107.4° and 96.5°, respectively.
Other Information on Thionyl Chloride
1. Synthesis of Thionyl Chloride
Thionyl chloride is obtained by distillation of phosphoryl chloride (POCl3), which is produced by the reaction of phosphorus pentachloride (PCl5) with sulfur dioxide (SO2). Thionyl chloride can also be produced by the reaction of sulfur trioxide (SO3) with sulfur dichloride (SCl2).
Instead of sulfur trioxide, thionyl chloride can be produced by oxidation with fuming sulfuric acid (Oleum) or chlorosulfonic acid (ClSO3H). The reaction is easier when a catalyst such as antimony chloride is used.
Thionyl chloride can also be obtained by reacting a mixture of sulfur dioxide (SO2) and sulfur tetrachloride (SCl4) using an activated carbon catalyst and distilling the product.
2. Reaction of Thionyl Chloride
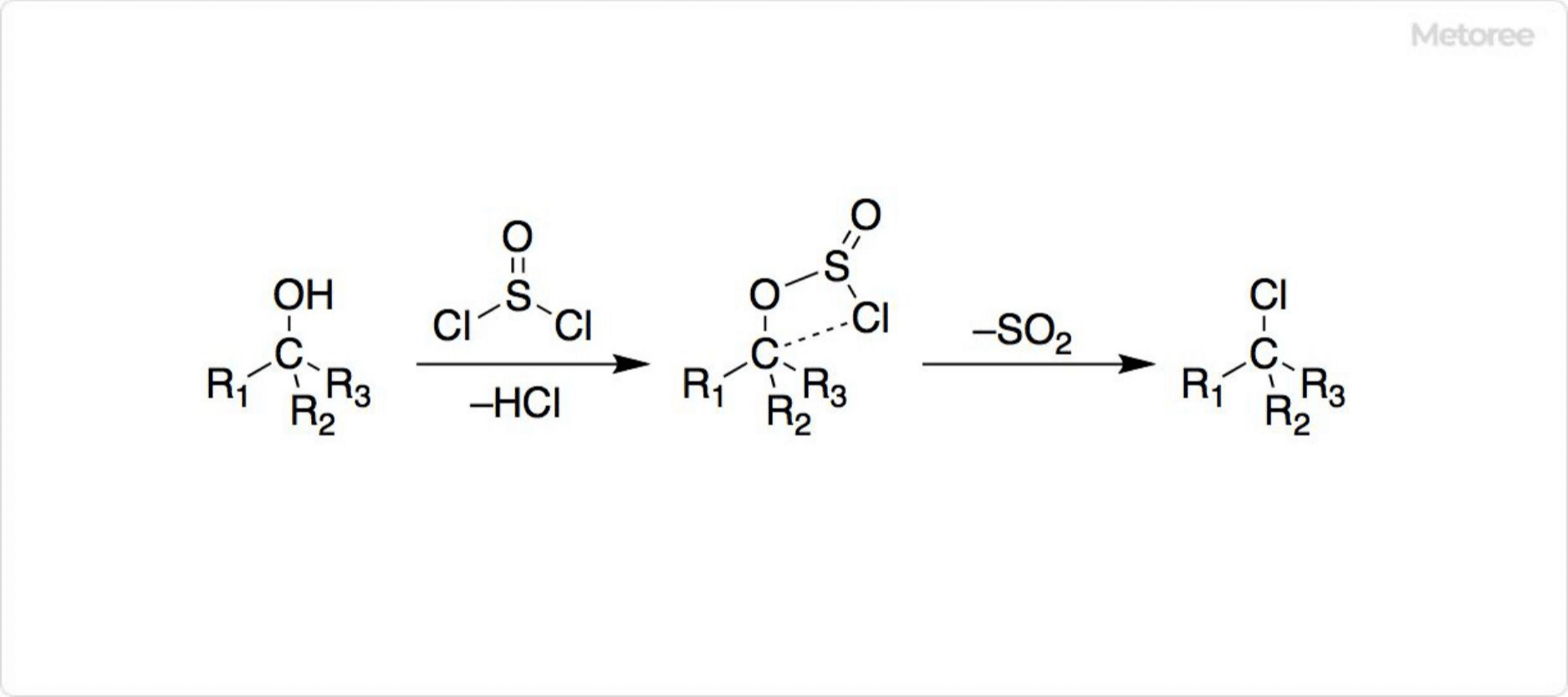
Figure 3. Reaction of Thionyl Chloride With Alcohol
Thionyl chloride is often used to chlorinate carboxylic acids and alcohols. Unlike other halogenating agents, the products of the reaction are gases such as HCl and SO2, and thionyl chloride has a low boiling point, making it easy to remove from the reaction system.
In addition to that, chlorination of alcohols with thionyl chloride does not proceed in the SN1 or SN2 reactions as with other chlorinating agents. Therefore, the reaction occurs in a sterically preserved manner without Walden inversion. A four-membered ring transition state has been proposed as the reaction mechanism and is called the SNi mechanism.
3. Storage of Thionyl Chloride
It is recommended that thionyl chloride be stored in a cool, dark, well-ventilated place with the container tightly closed and away from moisture and direct sunlight. Hydrolysis occurs when exposed to moisture, and hydrochloric acid is formed when reacting violently.