What Is a pH Indicator?
A pH indicator is a generic term for organic dyes that change color with changes in pH. They are also called acid-base indicators or neutralizing indicators. The pH at which the reaction that changes the structure of the indicator occurs differs depending on the indicator. Therefore, it is important to select an indicator that matches the pH range to be checked. Phenolphthalein, which changes colors in basic solutions, is a typical pH indicator.
Most pH indicators are often used in R&D and production in the manufacturing industry and are also used to check the pH of crops and soil. In addition, pH indicators have long been used in school education, such as science classes, because of their large color change and ease of use.
Uses of pH Indicators
Since pH indicators can easily determine the pH of a sample solution, they are widely used, especially in analytical applications. For example, in the manufacturing industry, they are often used at production sites to check the neutralization point or to check the pH of wastewater. In particular, the pH range of wastewater discharged from factories to the sewage system is set by the government, so pH control of the wastewater is essential.
In addition, it is sometimes used to check the pH of soil and food in agriculture. In particular, pH measurement is extremely important for stable crop growth because soil pH has a significant impact on how crops grow, and the optimal pH varies depending on the crop and the original pH of the soil in different regions.
Principle of pH Indicators
The color of the pH indicators changes because the chemical structure of the pH indicators changes with changes in pH. The change in the aromatic ring and the position of the double bond changes the energy state of the molecule (HOMO and LUMO energies), resulting in a change in the wavelength of the light absorbed.
1. BTB Solution
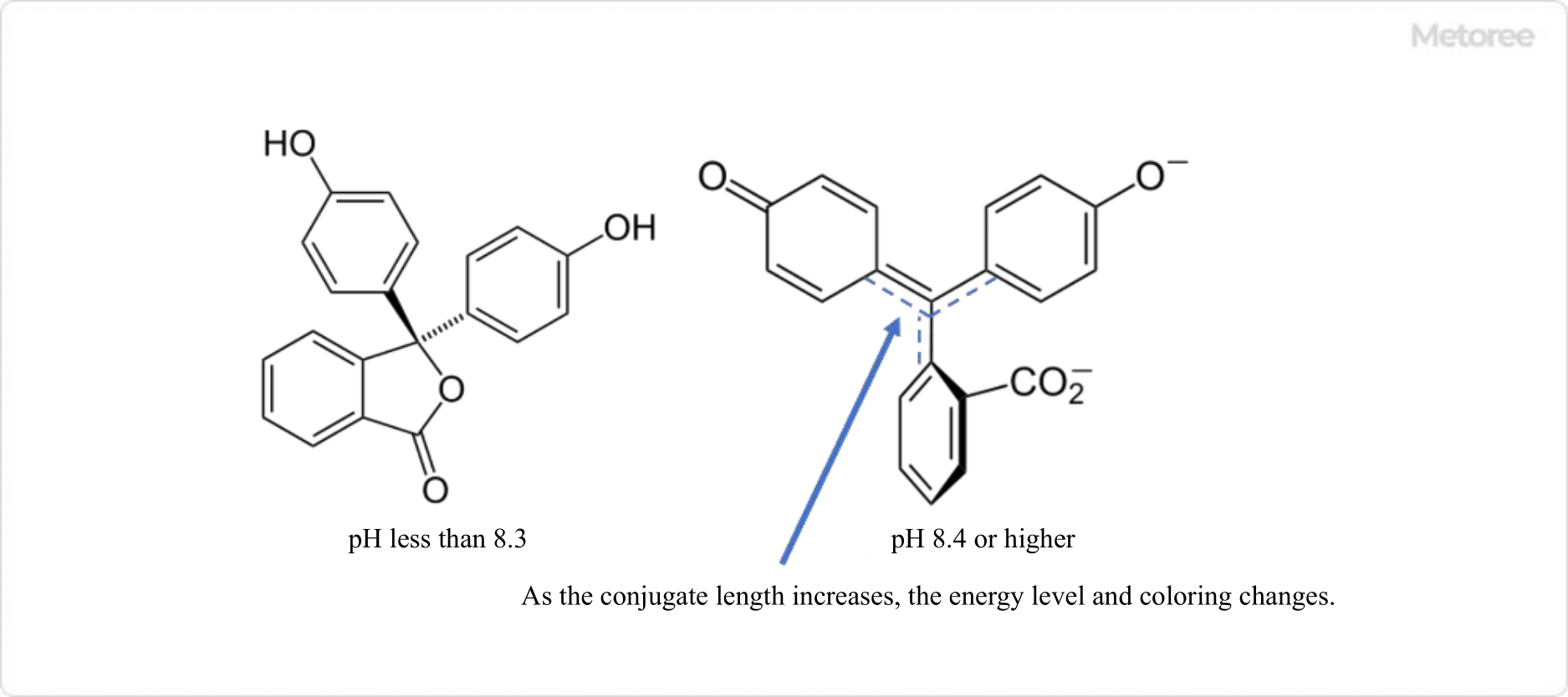
Figure 1. Structural changes in BTB
BTB solution (bromothymol blue) is used for pH indicators to distinguish between acid and base because the color of BTB varies greatly among acidic, neutral, and basic solutions. Strictly speaking, BTB is acidic, so the chemical structure of commercial products is sodium salt.
When the pH of a BTB solution is less than 6.0, almost all BTB forms a salt with sodium, but when the pH is greater than 7.6, the proton of the hydroxy group of BTB is also desorbed to form a divalent anion. This structural change results in a color change: yellow for pH less than 6.0, blue for pH greater than 7.6, and green for pH in between, which is a superposition of the colors of the two compounds.
2. Phenolphthalein
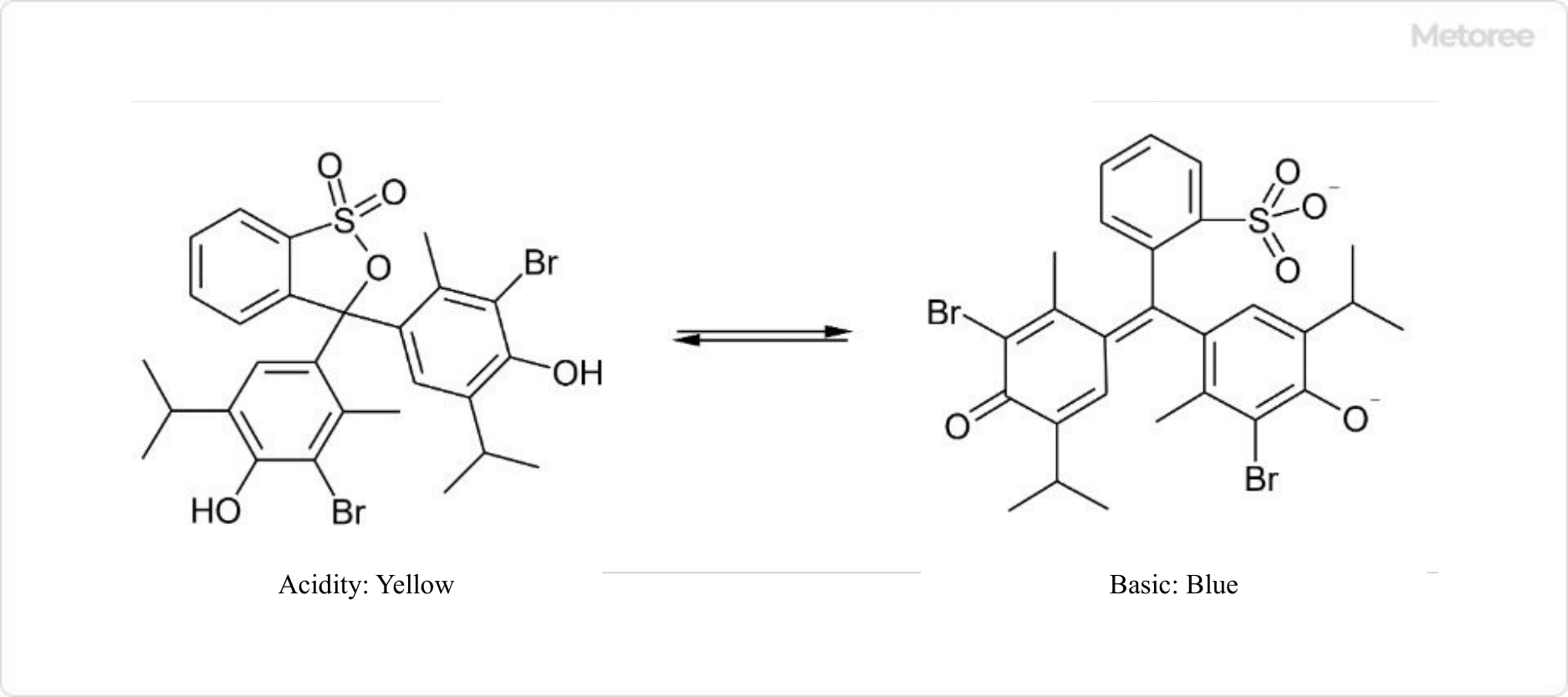
Figure 2. Structural changes in phenolphthalein
Phenolphthalein solution in pH indicators is used to identify basic solutions. It is often used in school science experiments because of its easily recognizable purple coloration.
Phenolphthalein is a white solid substance that is soluble in ethanol and water. Phenolphthalein solutions in neutral or acidic environments are clear, but when the pH exceeds 9, the ether moiety cleaves and changes to an anion, turning the solution purple. On the other hand, above pH 10, phenolphthalein undergoes further structural changes, and above pH 13, the purple solution returns to clear and colorless.
Other Information on pH Indicators
1. pH Indicators in the Manufacturing Industry
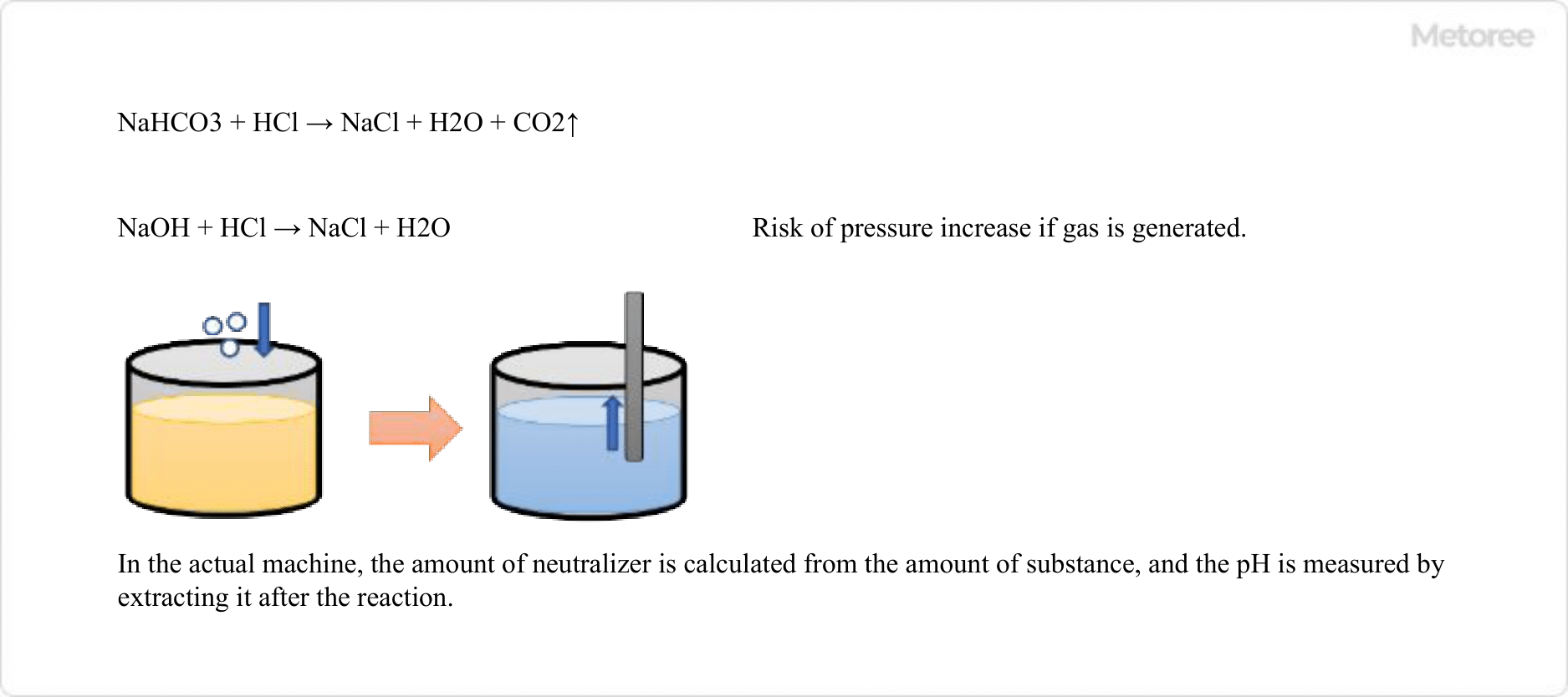
Figure 3. pH indicator use in the manufacturing industry
The pH of wastewater that can be discharged into the sewage system by businesses is set by each municipality and must be neutral. In cases where the amount of wastewater used is small, such as in laboratories and R&D, a weak base such as sodium bicarbonate is used as a neutralizing agent, and pH indicators, which change color near neutral, are added to the wastewater to check its pH.
However, neutralization using sodium bicarbonate generates carbon dioxide gas, which in some cases cannot be used at production sites on large scales. In such cases, neutralization may be performed using a base or acid such as hydrochloric acid or sodium hydroxide that does not generate gas.
Since the indicator cannot be added directly to the wastewater at the production site, the wastewater is sampled after treatment and the indicator is added to check the pH level.
2. Use of pH Indicators in Agriculture
In agriculture, soil pH is controlled due to its significant impact on crops. In addition, pH confirmation is often required not only for crops but also for a wide range of food products.
For example, for foods that dissolve in water, such as dried soup, pH indicators may be added to the food dissolved in water for simple analysis. For solid foods such as meat and fish, a pH measuring instrument using an electrode is used instead of pH indicators.