What Is a Spectrofluorometer?
A spectrofluorometer is an instrument that analyzes the light emitted from molecules and ions in a sample.
It is a type of spectrophotometer. Other types of spectrophotometers include UV/visible spectrophotometers and infrared spectrophotometers. Since the emission spectrum differs for each molecule and ion, it is possible to quantify the components contained in a sample based on the wavelength and intensity of the emission peak.
A spectrofluorometer is extremely sensitive and is used to detect trace elements. In biochemistry, it is also used to analyze the movement of proteins in living organisms by combining it with fluorescent probes that bind to specific compounds.
In samples containing multiple components, such as living organisms and foods, the luminescence of each component overlaps, resulting in complex spectra. Recently, statistical analysis methods, such as multivariate analysis, are being considered to extract information on a large number of components.
Uses of Spectrofluorometers
Spectrofluorometers are used to detect and quantify the smallest amounts of components in a sample. Quantitative analysis by spectrofluorometry is 1,000 times more sensitive than absorption spectrophotometry.
Specifically, they are used to measure the quantum yield, which is an indicator of the luminous efficiency of white LEDs and organic EL elements, as well as to analyze the spectrum of light emitted by the elements. Spectral analysis is extremely complex, but analysis software is becoming more sophisticated and can extract various information.
Principle of Spectrofluorometers
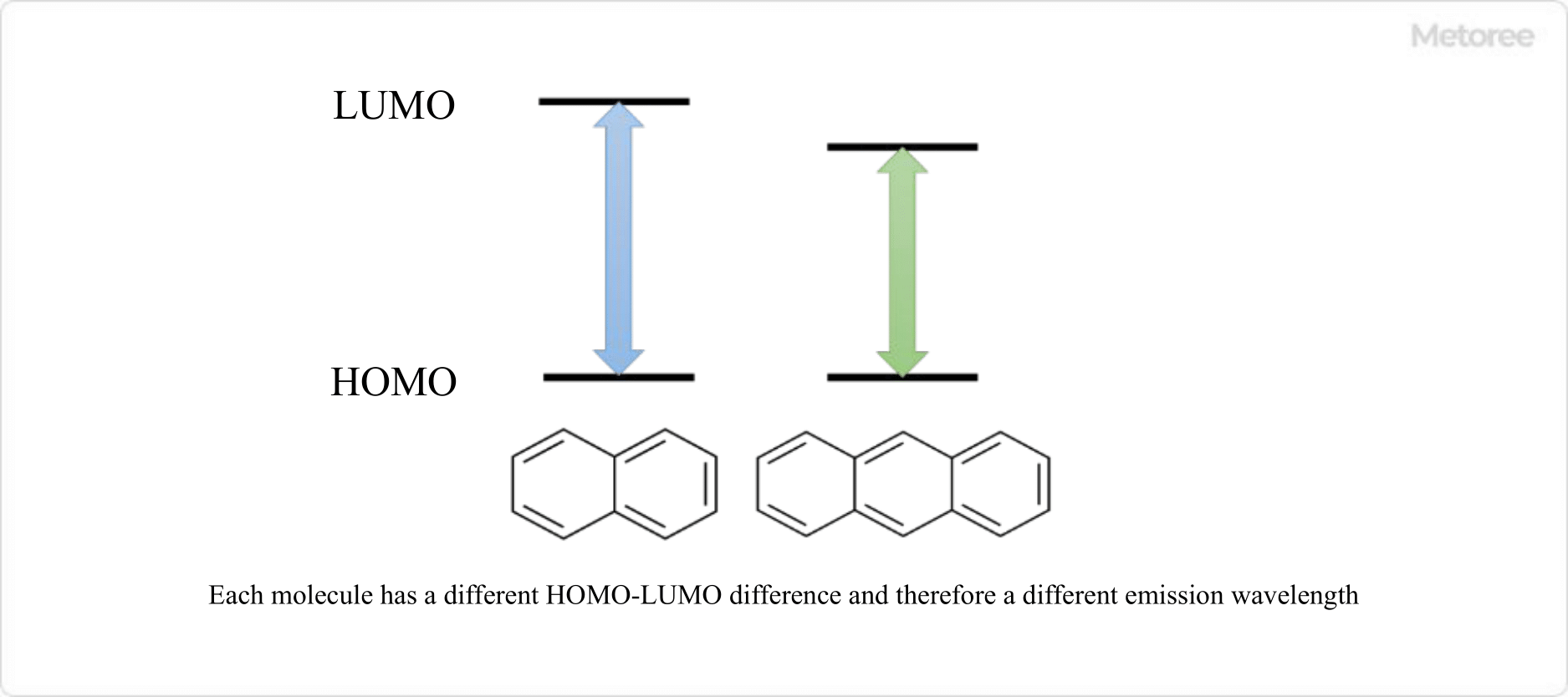
Figure 1. Principle of spectrofluorometerol
Spectrofluorometers are instruments that use fluorescence (or phosphorescence). This is the extra energy emitted as light when electrons in molecules and ions return from their excited state to their ground state. Each molecule has its own unique energy state and selectively absorbs light of a specific wavelength to transition to the excited state.
The electrons in the excited state immediately return to the ground state, emitting light with a wavelength corresponding to the difference in energy levels between the excited and ground states. If the irradiated light is not of a wavelength that is absorbed by the sample, no fluorescence is emitted and the measurement cannot be performed.
Other Information on Spectrofluorometers
1. Spectrofluorometers and Multivariate Analysis
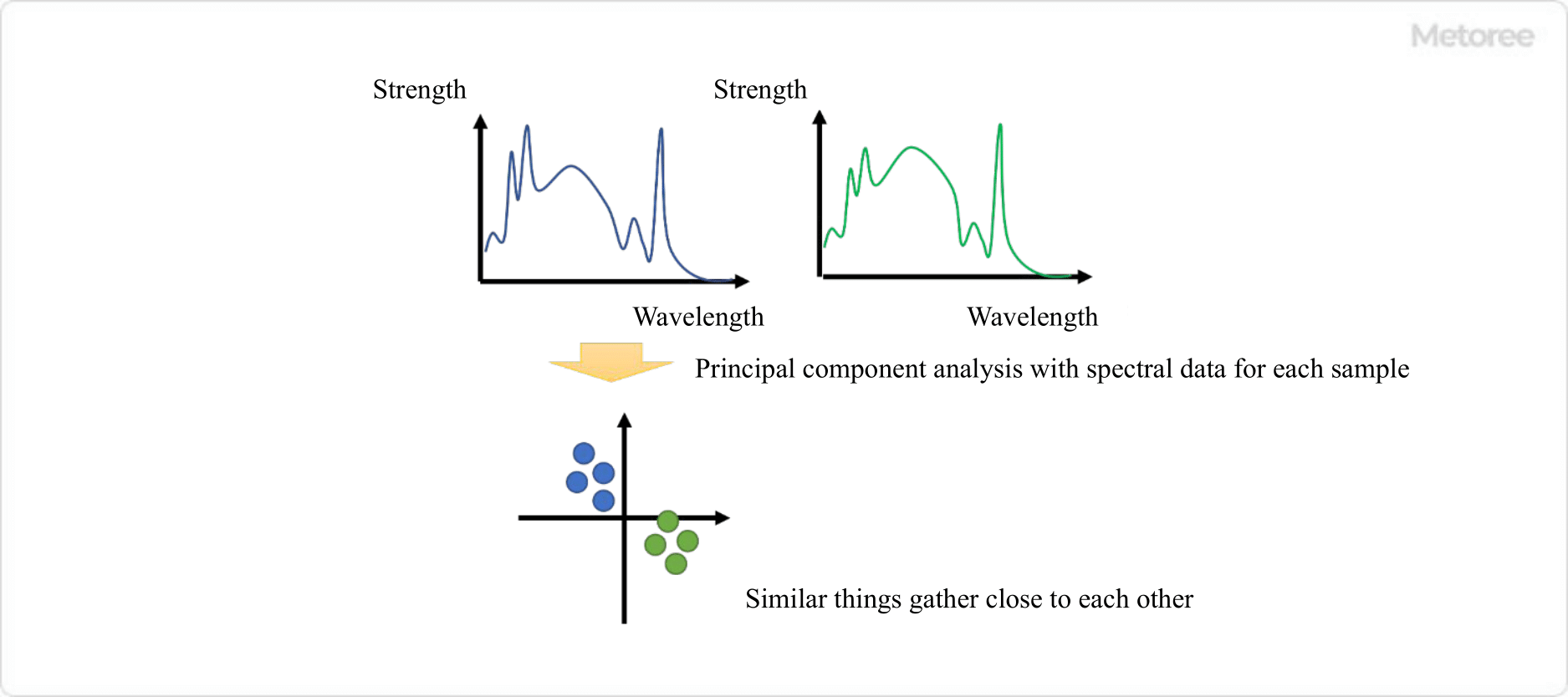
Figure 2. Spectrofluorometer and multivariate analysis
Fluorescence measurement of samples containing many organic substances, such as food, has been used to analyze the classification of patterns by origin and raw material. When a sample contains multiple components, the spectrum obtained by spectrofluorometers is the sum of the fluorescence emitted by each component.
In general, the fluorescence spectrum of a sample containing multiple components is very complex and difficult to analyze. In particular, samples containing numerous organic substances, such as food and beverages, will have many peaks, which can only be analyzed by a skilled person.
On the other hand, there have been recent attempts to obtain information from complex emission spectra of foods using multivariate analysis and statistical analysis methods. For example, principal component analysis (PCA), one of the multivariate analysis methods, can be used to compress multidimensional data such as spectra into two or three lower dimensions.
After the PCA is performed, the distribution of each sample is used for grouping analysis.
2. Application of Spectrofluorometers in the Field of Biochemistry
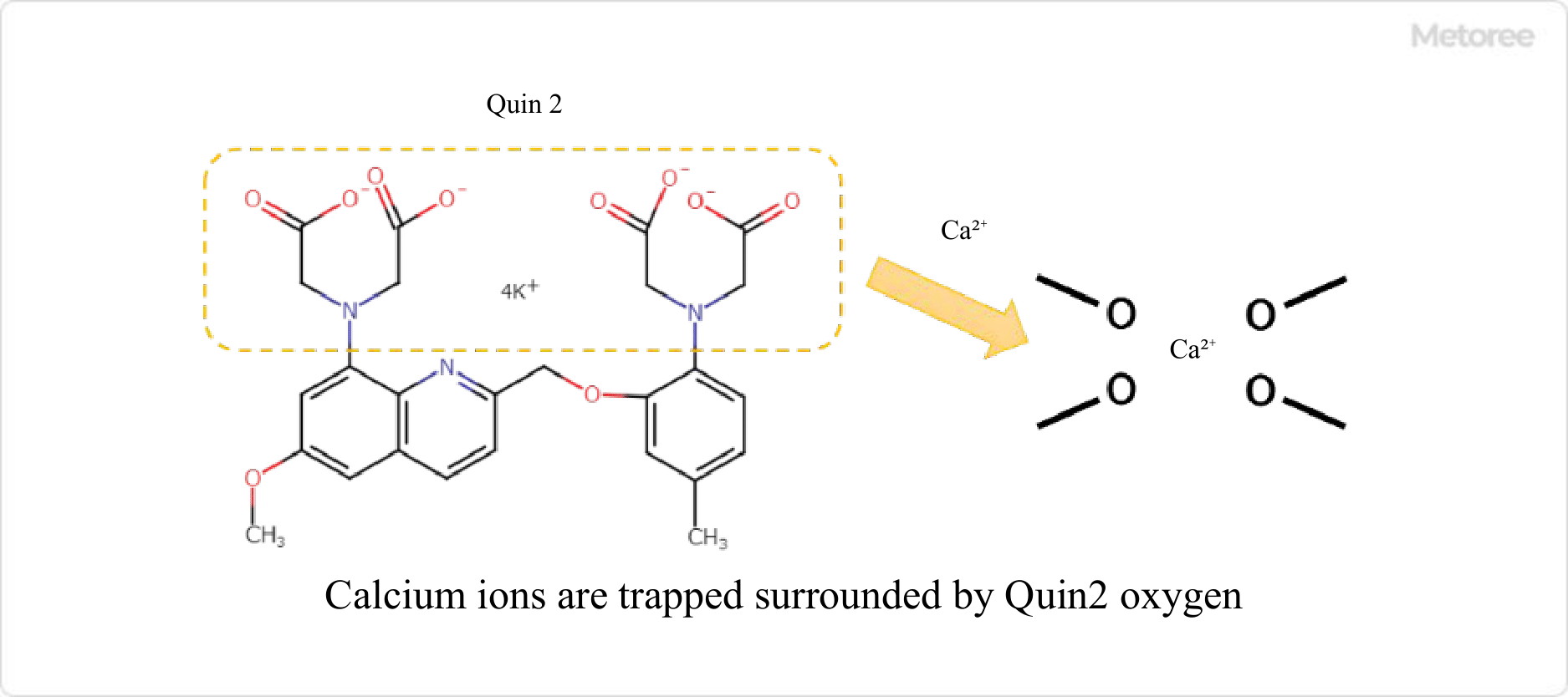
Figure 3. Utilization of spectrofluorometer
In the field of biochemistry, fluorescent probes can be selectively bound to specific proteins or calcium ions, enabling the quantification of the relevant components. For example, in the detection of calcium ions, a compound called a chelating agent, which has a structure that selectively traps ions, can be used.
Other fluorescent probes include polymers modified from fluorescent proteins derived from living organisms. These polymers are derived from fluorescent proteins, and their introduction enables them to replicate in living cells themselves.
The Nobel Prize-winning Japanese scientist Osamu Shimomura is credited with the discovery of this green fluorescent protein. The ability to introduce fluorescent proteins into biomolecules and to detect them with high sensitivity using a fluorometer has greatly advanced the analysis of biomolecules.