What Is a Mass Spectrometer?
Figure 1. Image of a mass spectrometer
A mass spectrometer (MS) is an instrument that ionizes molecules within a sample and detects and identifies the mass-to-charge ratio (m/z) of the resulting ions.
The abbreviation “MS” is used internationally. When molecules are ionized by an ionization method, they are propelled by electrostatic forces.
A mass spectrometer is an analytical instrument that separates and detects ions in motion based on their mass-to-charge ratio (m/z) through electrical, magnetic, or other actions in a vacuum. The instrument primarily comprises a sample introduction section, an ion source, a mass separation section, and a detector.
There are several types depending on the ionization and mass separation methods, and they are used according to the measurement sample and application. Mass spectrometers are mainly used for sample identification, component analysis of unknown samples, and distinguishing and detecting isotopes.
Uses of Mass Spectrometers
Mass spectrometers are utilized for qualitative and quantitative analysis of a wide range of molecules, from low-molecular-weight compounds to high-molecular-weight compounds such as proteins and synthetic polymers.
Due to its effectiveness in identifying known substances and determining the structure of unknown substances, mass spectrometry is widely used in organic chemistry, biochemistry, and other chemical and biological fields. Specifically, it finds applications in research and development, quality control, analysis, and testing of various agricultural chemicals, pharmaceuticals, and naturally occurring compounds.
Recent advancements have enabled the ionization of proteins with large molecular weights, extending the use of mass spectrometers to the fields of life sciences and medicine.
Principle of Mass Spectrometers
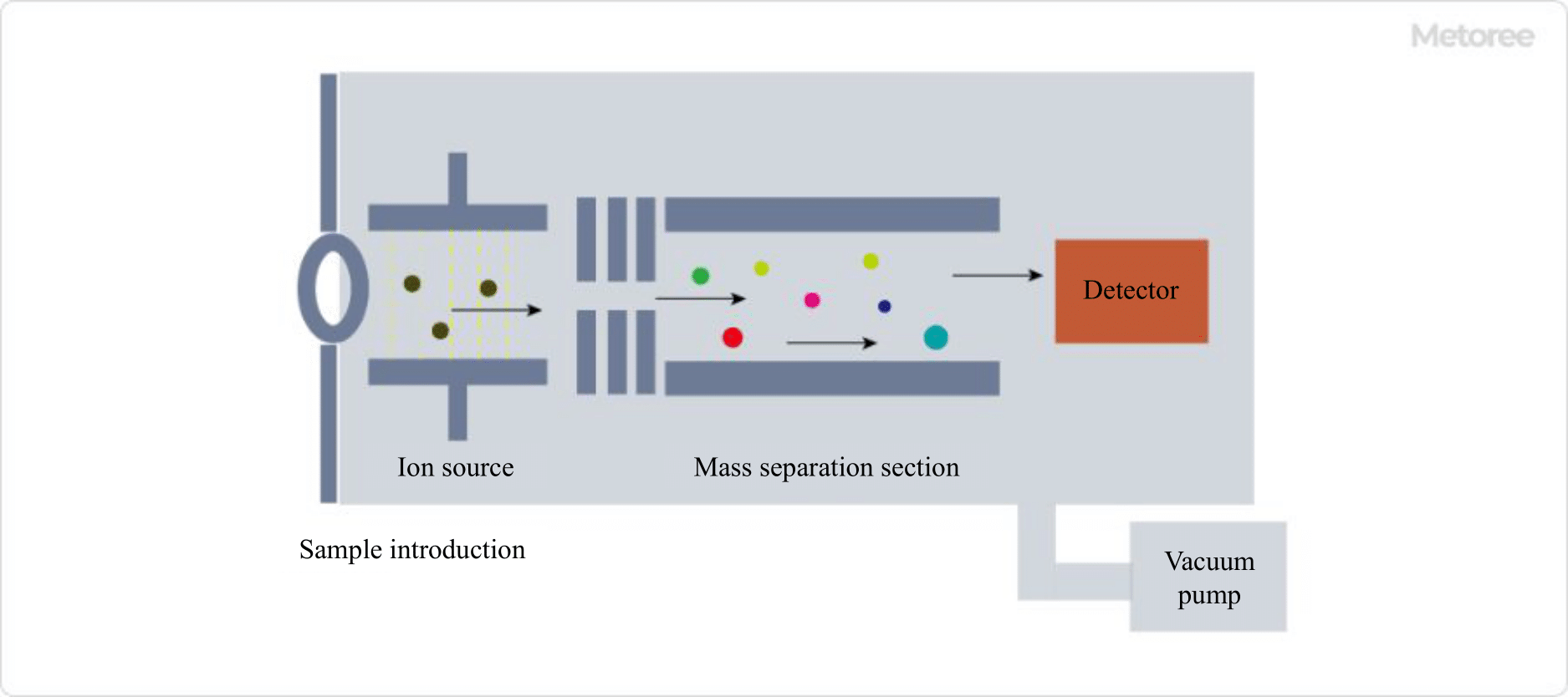
Figure 2. Principle of a mass spectrometer
The fundamental principle of a mass spectrometer involves the following series of steps, with the mass spectrum displayed using m/z as the abscissa and detection intensity as the ordinate:
- The sample is introduced into the instrument through the sample introduction section.
- The sample is ionized by the ion source.
- In the mass separation section, the sample is separated based on the different effects of magnetic and electric fields on ions according to their m/z, and detected by the detector.
Mass spectrometers can detect single-charged ions, which have only one charge, as well as multiply charged ions, fragment ions resulting from dissociation, or aggregate ions formed by the association of samples with each other. Peaks typically exhibit a unique distribution derived from the isotopic ratio of the original molecule.
Types of Mass Spectrometers
There are various types of mass spectrometers classified primarily by the combination of the type of ion source and the type of mass separator. Examples include “MALDI-TOF-MS” and “ESI-TOF-MS.”
1. Sample Introduction Section
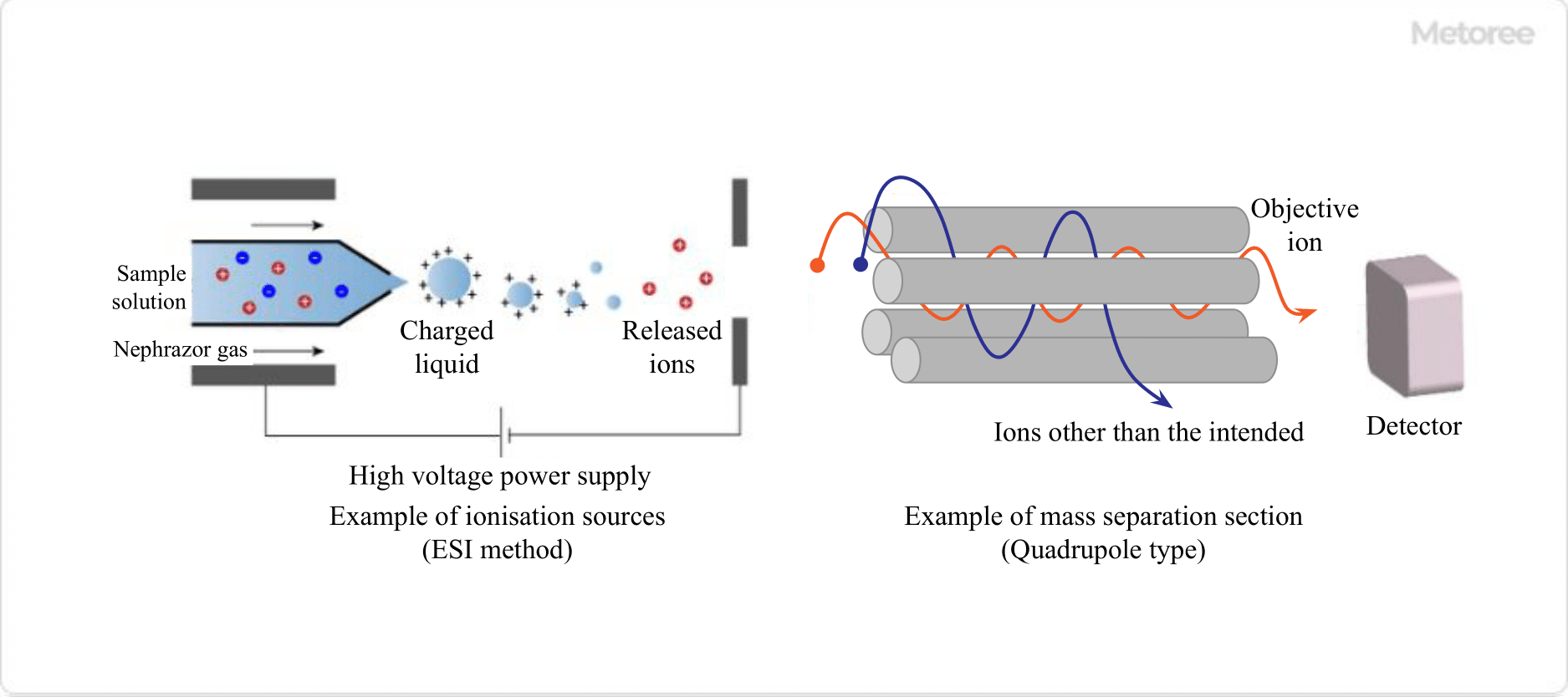
Figure 3. Example of ionization source and mass separation part
Some mass spectrometers are combined with other instruments before the sample introduction section and are used in research and development and quality control. Examples include LC-MS combined with liquid chromatography, GC-MS combined with gas chromatography, and ICP-MS combined with inductively coupled plasma.
2. Ion Source
Electron Ionization (EI) Method
Accelerated electrons collide with thermally vaporized molecules (M) in a high vacuum. The electrons are then ejected from the molecule, producing radical cations (M+) called molecular ions.
Electrospray Ionization (ESI) Method
- First, a sample solution is introduced into a capillary to which a high voltage is applied.
- Atomizing gas (nebulizer gas) flows from the outside of the capillary to spray it, forming charged droplets.
- As the charged droplets move, the solvent evaporates and the surface electric field increases, eventually causing the droplets to split when the repulsive force between the charges exceeds the surface tension of the liquid.
- This process releases sample ions into the gas phase.
Matrix-Assisted Laser Desorption Ionization (MALDI) Method
This method involves mixing a sample with a matrix, such as an aromatic organic compound, to form a crystal. The crystal is then ionized by irradiating it with a laser. It is suitable for ionizing high-molecular-weight compounds such as proteins.
Fast Atom Bombardment (FAB) Method
This method ionizes sample molecules by high-speed collisions with neutral atoms in the presence of a matrix and a sample solution dissolved in an organic solvent.
Other methods include CI, FD, APCI, and ICP methods.
3. Mass Separation Section
Quadrupole (Q)
This method uses four electrode rods to apply a high-frequency voltage to ions from an ion source. The electrodes are subjected to DC and AC voltages to create an electric field that allows ions with a specific m/z to reach the detector.
This method can measure ions in the desired m/z range up to approximately m/z 4000.
Double-Focusing Type
This is a magnetic sector-type mass separator. In magnetic sector-type separators, ions pass through a magnetic field, altering their flight paths due to the Lorentz force. The double-focusing type combines magnetic and electric field sectors to achieve both velocity and directional convergence of ions.
Time-Of-Flight (TOF)
This method accelerates ionized samples with known electric field strength and detects the time difference between the arrival of each ion at the detector. It can measure ions across a wide mass range.
Other methods include Ion Trap (IT), Fourier-Transform Ion Cyclotron Resonance (FT-ICR), and Accelerator Mass Spectrometry (AMS).