What Is a Mercury Lamp?
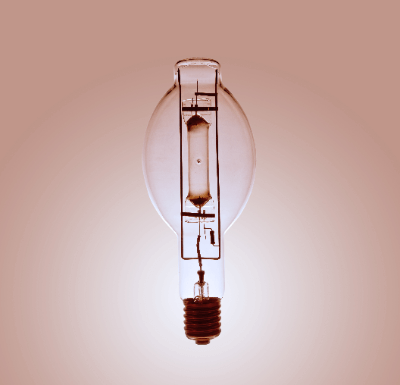
Mercury lamps are lamps that emit blue-white light using mercury vapor. Compared to incandescent lamps, mercury lamps have the advantages of higher luminous efficiency, longer life, and less maintenance.
Mercury lamps are broadly classified into two types based on the mercury vapor pressure at the time of lamp lighting: high-pressure and low-pressure. If the mercury vapor pressure is 10^5 Pa or higher, it is a high-pressure type, and if it is 100 Pa or lower, it is a low-pressure type.
Among high-pressure mercury lamps, there are also ultra-high-pressure types with mercury vapor pressures of 10^6 to several 10^7 Pa.
Uses of Mercury Lamps
Low-pressure mercury lamps are widely used as germicidal lamps because of their property of radiating ultraviolet (UV) light. Low-pressure mercury lamps may also be used as fluorescent lamps by coating the emission tubes with a fluorescent substance, in which case they are used for general lighting and as light sources for UV curing.
Typical applications for high-pressure mercury lamps include general lighting and UV curing. They are also sometimes used for photochemical reaction experiments.
There are two main types of super high-pressure mercury lamps: short-arc type and long-arc type. The former is used for optical microscopes and optical equipment due to its high luminance, while the latter is utilized for plate-making and semiconductor etching.
Principle of Mercury Lamps
Mercury lamps are designed to emit light by filling the light-emitting tube with mercury vapor and discharging the vapor into the mercury vapor.
When a discharge occurs in the light-emitting tube, mercury atoms in a low-energy state collide with electrons, resulting in a high-energy state (excited or ionized state). When the mercury atoms in this high-energy state return to their low-energy state, light equivalent to the difference in energy between the two is emitted.
The light emitted when the mercury ion returns to the mercury atom is called the continuous spectrum. The light emitted when the mercury ion returns from the excited state to the ground state (or metastable state) is called the emission line spectrum.
It is well known that the wavelength of light emitted by mercury lamps varies depending on the vapor pressure of mercury sealed in the light-emitting tube. Specifically, a low mercury vapor pressure strongly emits light with wavelengths in the ultraviolet region, while a high mercury vapor pressure increases light with wavelengths in the visible region.
In other words, low-pressure Mercury Lamps emit more ultraviolet light, making them suitable for sterilization lamp applications.