What Is an Isovaleric Acid?
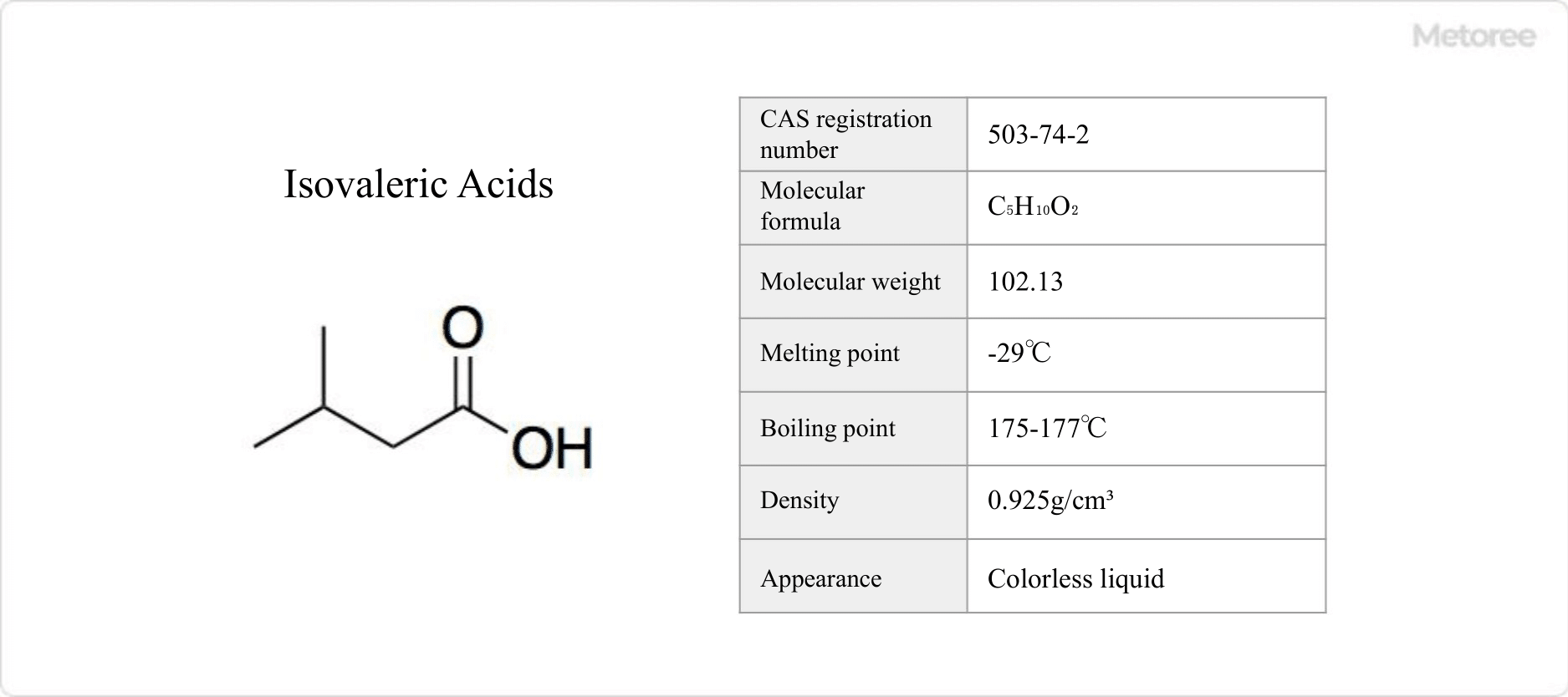
Figure 1. Basic information on isovaleric acids
Isovaleric acids are isomeric forms of valeric acid, an organic acid with the chemical formula C5H10O2.
It is also called 3-methylbutanoic acid. It has a pungent odor with an unpleasant sensation and is regulated as a specified malodorous substance under the Malodor Prevention Law. The ester of isovaleric acids, however, has a pleasant aroma.
Naturally, isovaleric acid is found in essential oils of plants such as cypress, geranium, rosemary, and lemongrass, as well as in fruits such as apples and grapes.
Uses of Isovaleric Acids
Isovaleric acids are a malodorous component of body odor, etc. Isovaleric acid ester has a pleasant aroma and is used as a flavoring component in non-alcoholic beverages and foods such as ice cream, baked goods, and cheese. It can also be used as a flavoring ingredient in perfumes.
Isovaleric acids are also widely used as a chemical intermediate in the manufacture of sedatives and other pharmaceuticals.
Additionally, it is used as an extractant of mercaptan from petroleum hydrocarbons, as a vinyl stabilizer, and as an intermediate in the manufacture of plasticizers and synthetic lubricants.
Properties of Isovaleric Acids
Isovaleric acids have a melting point of -29°C and a boiling point of 175-177°C. It is slightly soluble in water and dissolves well in many organic solvents.
Isovaleric acids are volatile. It is a liquid with an acidic, unpleasant, rancid cheese-like odor and is a causative agent of odors such as sweat, foot odor, and age-related odors.
Structure of Isovaleric Acids
Isovaleric acids have a molar mass of 102.13 g/mol and a density of 0.925 g/cm3. The specific formula is expressed as (CH3)2CHCH2CO2H. It is present at physiological pH in biological systems in the form of (CH3)2CHCH2COO–, the isovaleric acid ion.
Isovaleric acids are isomers of the branched structure of Yoshric Acid. Pivalic acid and hydroangelic acid also exist as structural isomers. Pivalic acid is also called pivalic acid trimethylacetic acid and neopentanoic acid.
Other Information on Isovaleric Acids
1. Synthesis of Isovaleric Acids
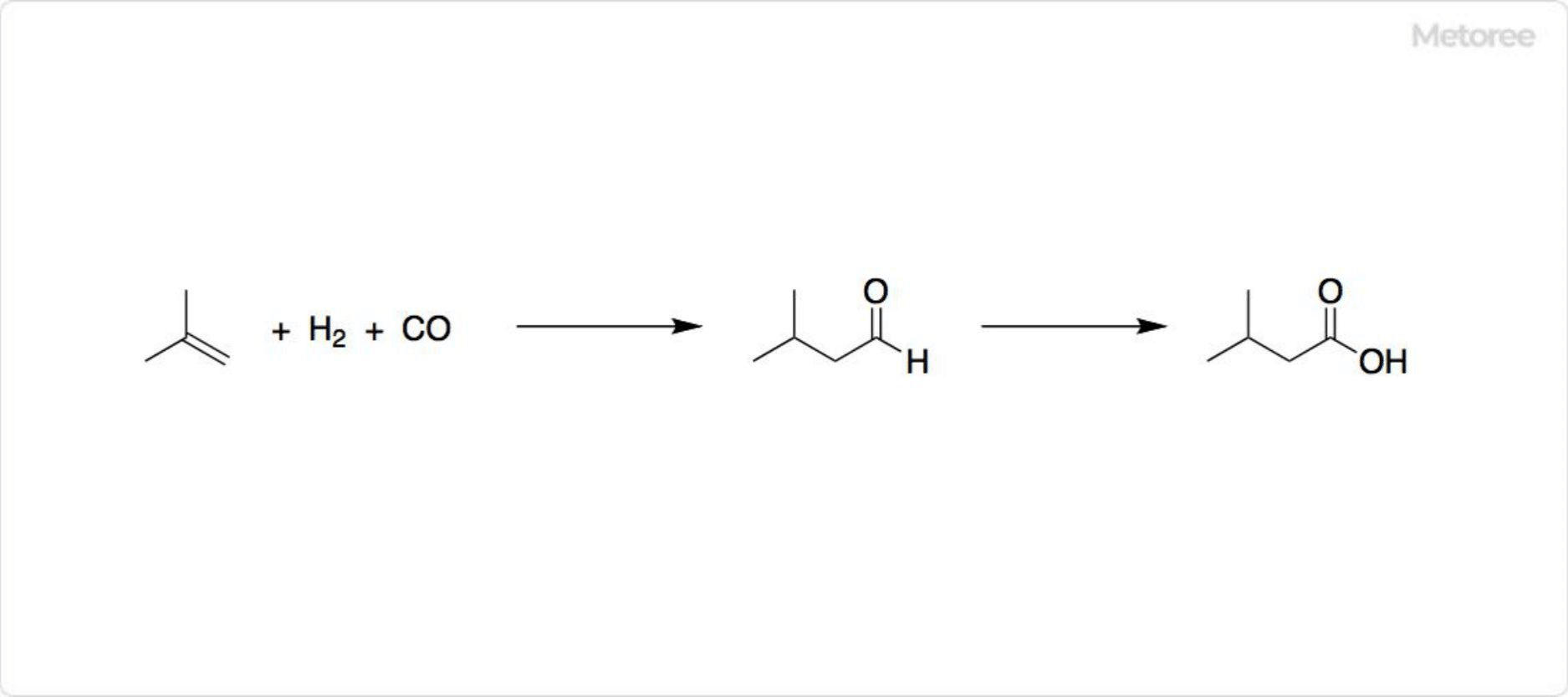
Figure 2. Synthesis of isovaleric acids
Isovaleric acids are a trace constituent of Valerian and are named isovaleric acids. The dried roots of Valerian have been used medicinally since ancient times, and research into isovaleric acids first began in the 19th century with the oxidation of components of fusel oil (Fusel alcohol) containing amyl alcohol.
Industrially, isovaleraldehyde is formed by the hydroformylation of isobutylene, which is oxidized to yield isovaleric acids.
2. Reaction of Isovaleric Acids
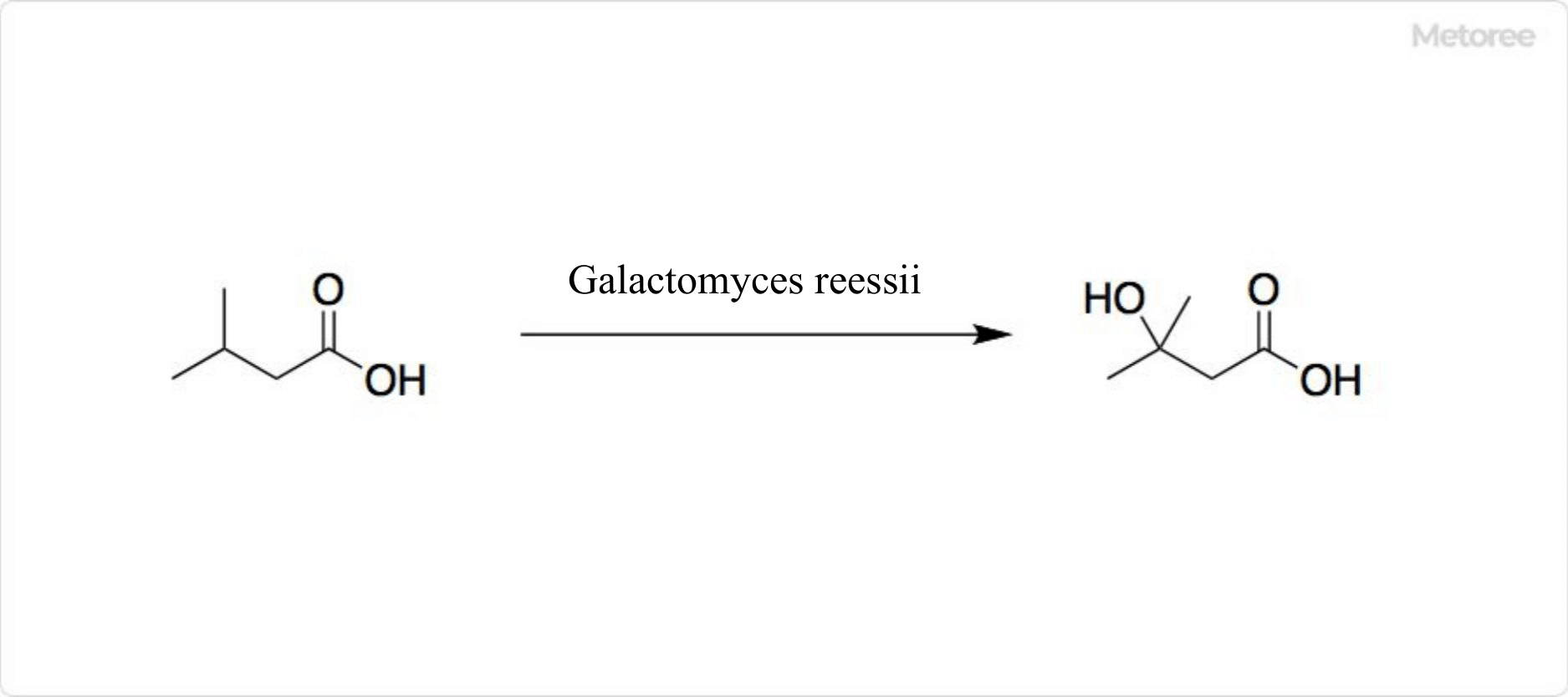
Figure 3. Reaction of Isovaleric Acids
In general, isovaleric acids react as carboxylic acids to form amides, esters, anhydrides, and chloride derivatives. Acid chlorides are widely used as synthetic intermediates.
Fermentation of isovaleric acid by the fungus Galactomyces reessii yields 3-hydroxyisovaleric acids. Hydroxy-β-methylbutyrate is another name for 3-hydroxyisovaleric acid.
3. Structural Isomers of Isovaleric Acids
Isovaleric acid is a linear carboxylic acid, a colorless liquid with the specific formula CH3(CH2)3COOH. It has a density of 0.94 g/cm3, a melting point of -34.5°C, and a boiling point of 186-187°C. It is the lowest molecular weight carboxylic acid soluble in non-polar solvents rather than polar solvents. It is a weak acid with a pKa of 4.82 and is corrosive to the human body.
Pivalic acid is a carboxylic acid with a tert-butyl group. It has a density of 0.905 g/cm3, a melting point of 35.5°C, and a boiling point of 163.8°C. It is a colorless liquid or white crystal with a pungent odor. pKa is 5.01 and the aqueous solution is slightly acidic.
The specific formula of hydroangelical acid is C2H5(CH3)CHCOOH. Hydroangelical acid has mirror isomers. It has a density of 0.94 g/cm3, a melting point of -90°C, and a boiling point of 176°C.