What Is an Isobutyric Acid?
Isobutyric acid is an organic compound with the chemical formula C4H8O2 and the characteristic formula (CH3)2CHCOOH.
It is one of the structural isomers of butyric acid, a volatile aliphatic carboxylic acid. Its IUPAC nomenclature is 2-methylpropanoic acid, and its CAS number is 79-31-2.
Uses of Isobutyric Acids
In the chemical field, isobutyric acids are used for the synthesis of isobutyric esters such as methyl isobutyrate, propyl ester, isoamyl ester, and benzyl ester, and the production of isobutyronitrile intermediate.
It is also used in the food industry as an edible flavor. Specifically, it is mainly used in the production of butter, apples, caramel, cheese, bread, and yeast. Other uses include the production of perfumes and perfume esters, pharmaceuticals, solvents for paints, disinfectants, varnishes, plasticizers, leather, and tanning agents.
Properties of Isobutyric Acids
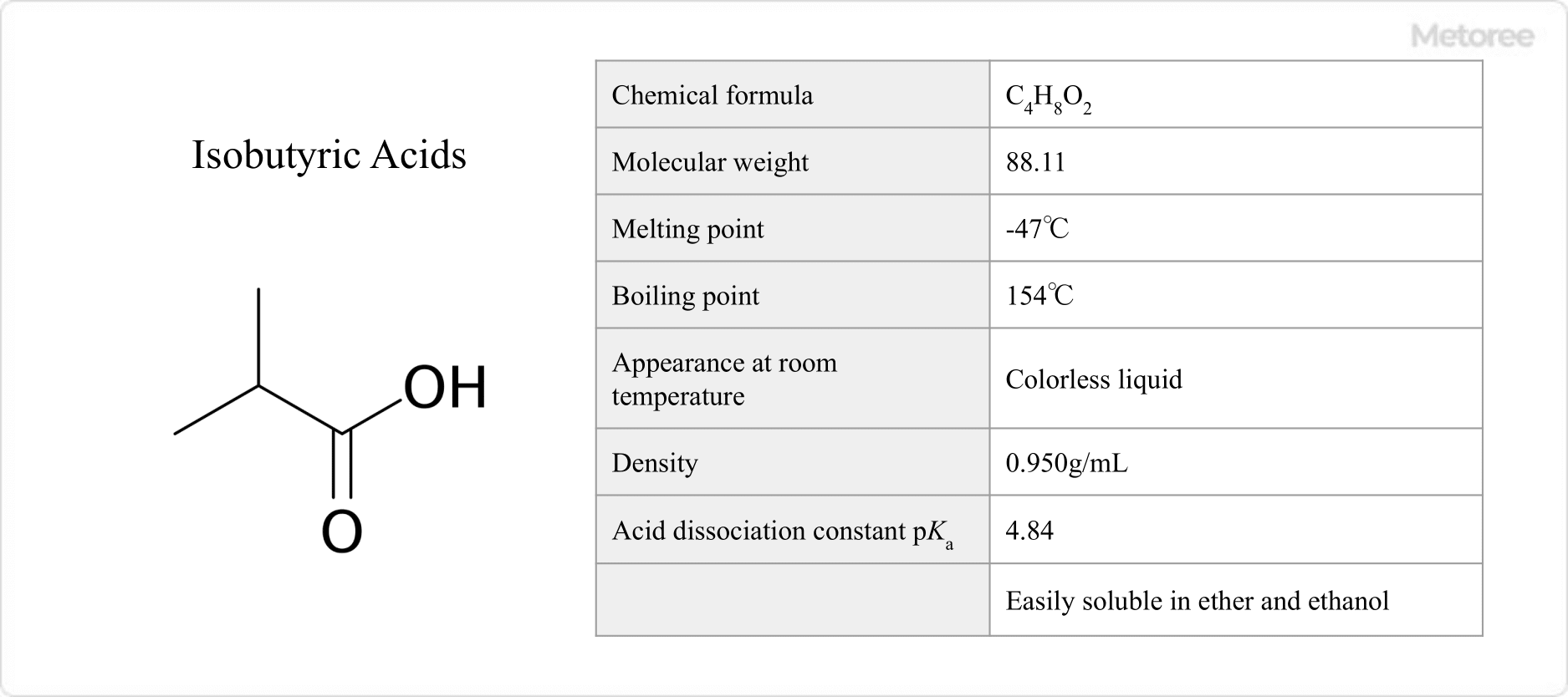
Figure 1. Basic Information on Isobutyric Acids
Isobutyric acids have a molecular weight of 88.11, a melting point of -47°C, and a boiling point of 154°C. It is a colorless liquid at room temperature. it has an unpleasant rancid buttery odor similar to that of n-butyric acid.
It has a density of 0.950 g/mL and an acid dissociation constant pKa of 4.84. It is extremely soluble in organic solvents such as ethanol and ether and dissolves in 6 times as much water.
Types of Isobutyric Acids
Isobutyric acids are generally sold as reagent products for research and development and as flavoring agents (food additives). In R&D reagent products, isobutyric acid is available in 25mL, 100mL, and 500mL volumes, and is usually supplied in volumes that are easy to handle in the laboratory.
They are handled as reagent products that can be stored at room temperature. For those sold as food additives or flavorings, individual inquiries to the manufacturer are required.
Other Information on Isobutyric Acids
1. Synthesis of Isobutyric Acids
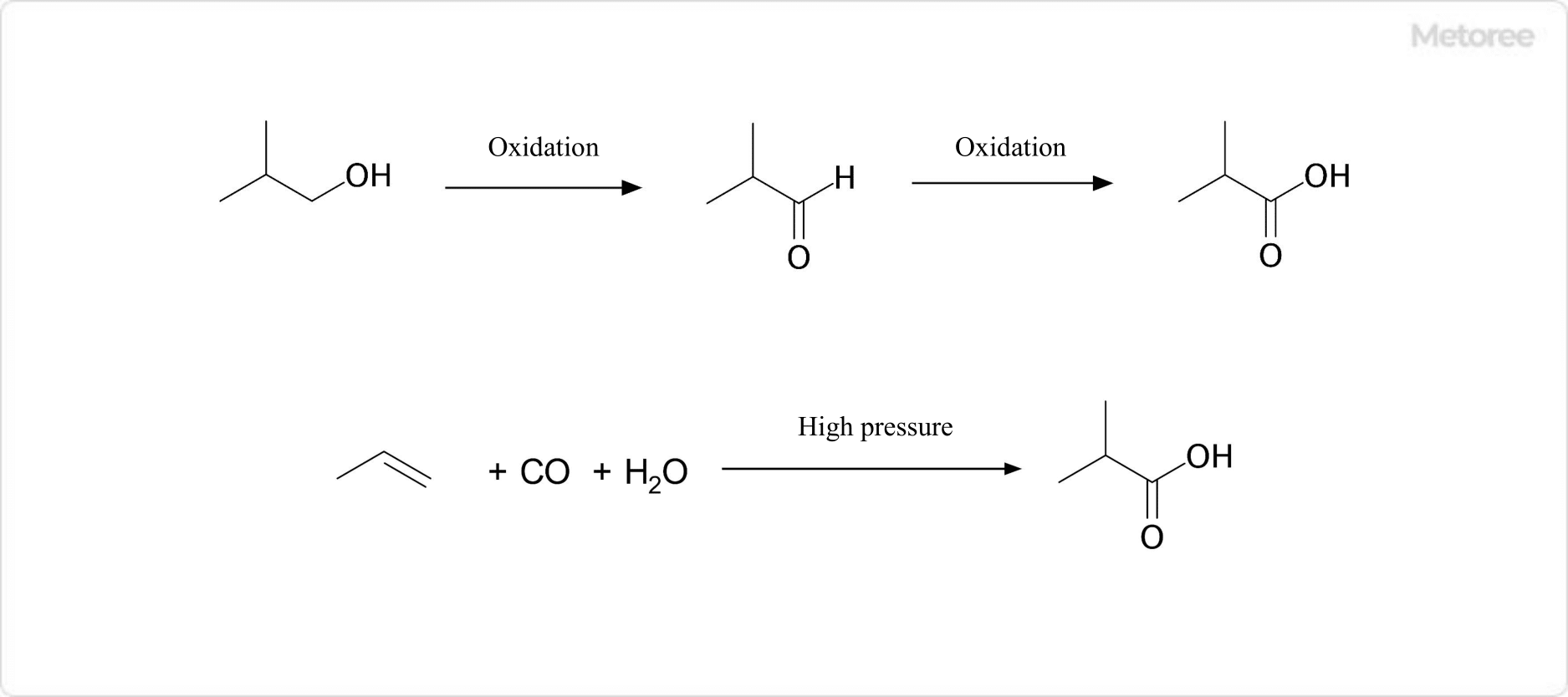
Figure 2. Example of Synthesis of Isobutyric Acids
Isobutyric acids can be synthesized by oxidizing isobutyl alcohol with an appropriate oxidant (potassium dichromate/sulfuric acid conditions, etc.). This is done via isobutyraldehyde as an intermediate.
Other methods include the hydrocarboxylation of propylene (Koch reaction). Industrially, isobutyric acids are a byproduct of n-butanol production.
Laboratory methods include hydrolysis of isobutyl nitrile under basic conditions to obtain isobutyl alcohol, followed by oxidation, and direct treatment of methacrylic acid with sodium amalgam (Na(Hg)) to obtain isobutyric acids.
2. Chemical Reaction of Isobutyric Acids
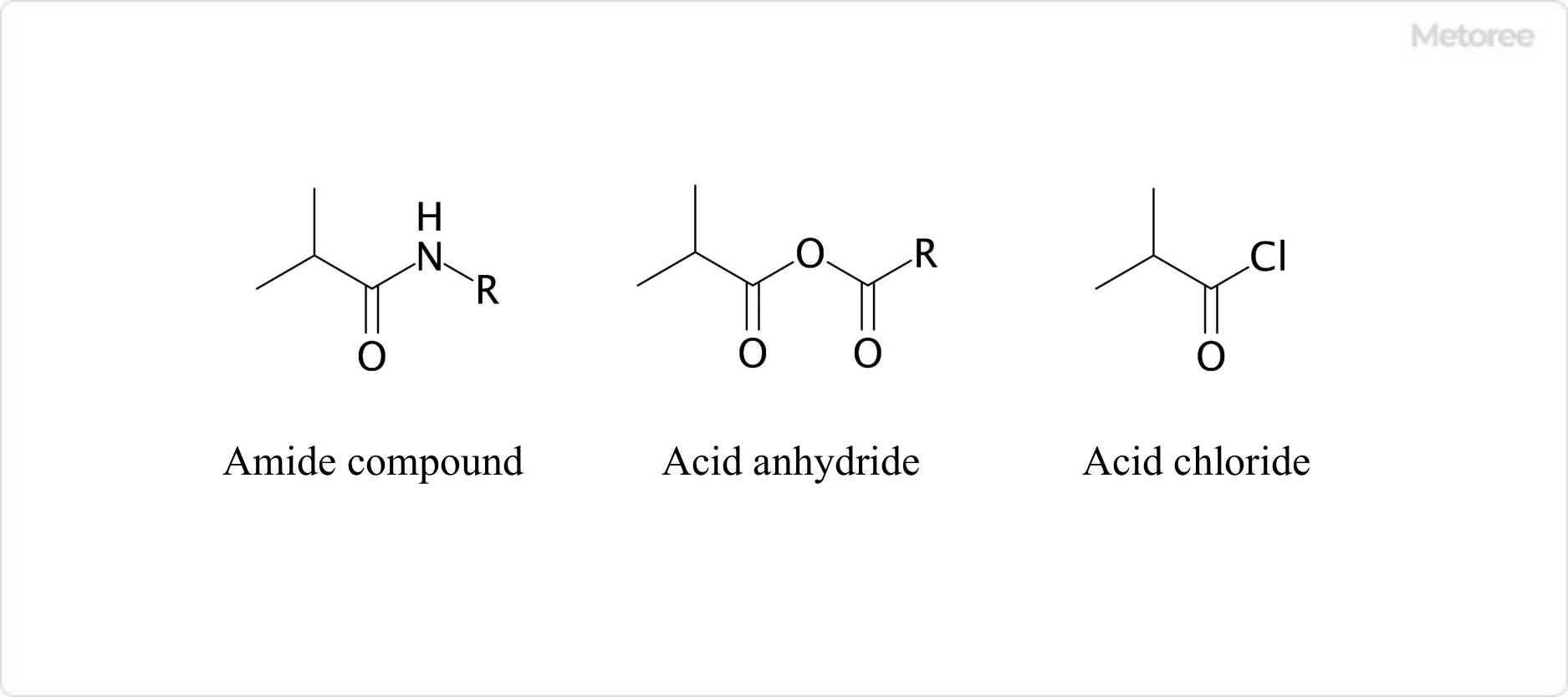
Figure 3. Examples of Derivatives of Isobutyric Acids
Isobutyric acids show the typical reactivity seen in carboxylic acids in general, yielding derivatives such as amides (-CONH2), acid anhydrides (-CO-O-CO-), and acid chlorides (-COCl). Reaction with chromic acid produces acetone. The substance obtained by oxidizing isobutyric acids with potassium permanganate under basic conditions is α-hydroxyisobutyric acid.
3. Hazardous Properties and Precautions for Handling Isobutyric Acids
Isobutyric acids have various hazardous properties and are classified by the GHS classification as follows
- Inflammable liquid: Category 3
- Acute toxicity (oral): Category 3
- Acute toxicity (dermal): Category 3
- Skin corrosion/irritation: Category 1
- Serious eye damage/eye irritation: Category 1
- Specific target organ toxicity (single exposure): Category 3
- Airway irritation: Category 3
- Hazardous to the aquatic environment (acute): Category 3
- Hazardous to the aquatic environment (chronic): Category 3
Isobutyric acids may be altered by light. Avoid high temperatures, direct sunlight, heat, flames, sparks, static electricity, and sparks. Strong oxidizers are listed as a miscibility hazard. Hazardous decomposition products are carbon monoxide and carbon dioxide.
4. Regulatory Information for Isobutyric Acids
Isobutyric acids are regulated by law due to their hazardous properties. Under the Fire Service Act, it is classified as a hazardous material, Class IV, Petroleum No. 2, Hazardous Rank III, and under the Industrial Safety and Health Act, it is classified as a hazardous material and flammable substance. It must be handled correctly in compliance with laws and regulations.