What Is Polycarbonate?
 Polycarbonate is an amorphous plastic with a molecular formula of C15H16O2. It is made from bisphenol A.
Polycarbonate is an amorphous plastic with a molecular formula of C15H16O2. It is made from bisphenol A.
It has outstanding impact resistance among resins, making it highly resistant to breakage, possessing high mechanical strength, transparency, non-toxicity, high weather resistance, and self-extinguishing properties. However, it has the drawbacks of low resistance to acids, alkalis, and solvents, and a susceptibility to scratching.
Polycarbonate is classified as a thermoplastic resin that softens when heat is applied.
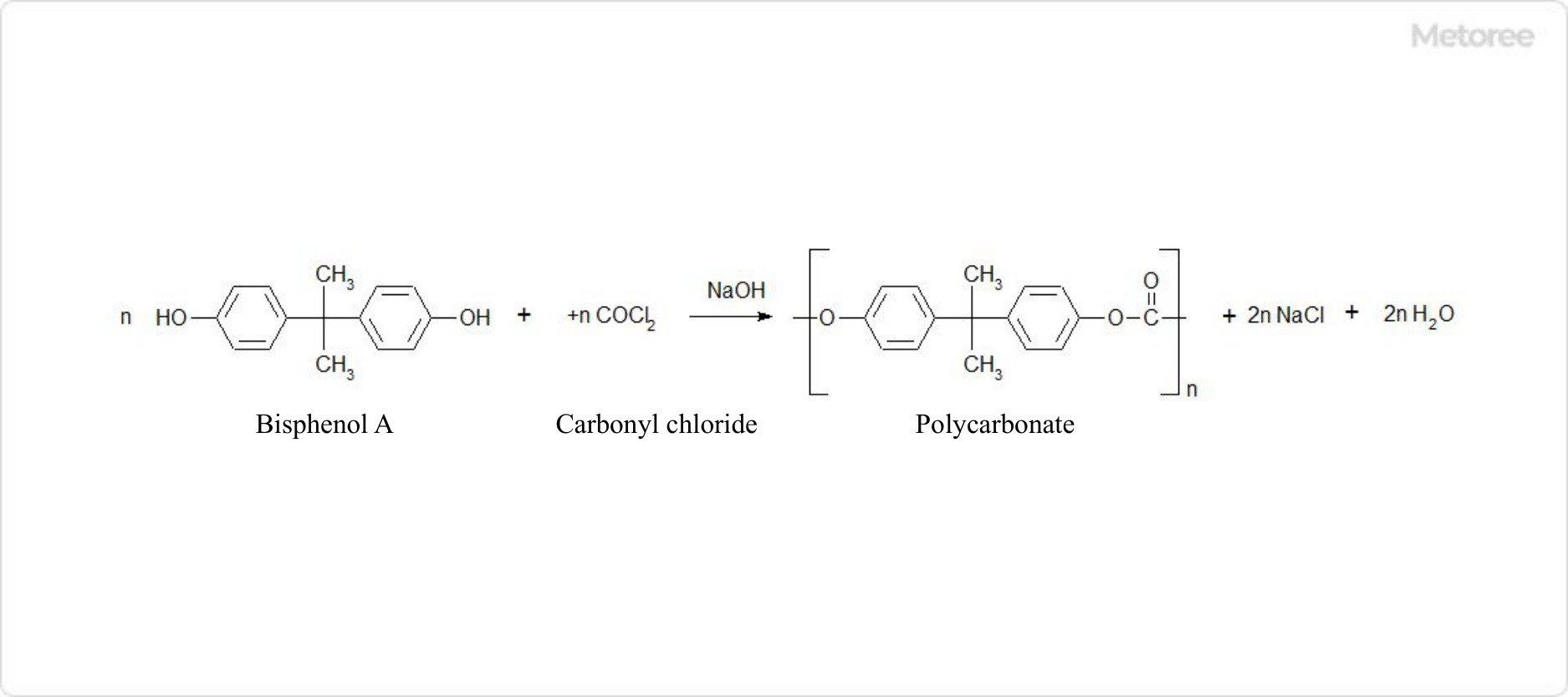
Figure 1. Polycarbonate Reaction Formula
Uses of Polycarbonate
Polycarbonate is used in a great many applications due to its resistance and processability.
1. Transparency
With transparency equivalent to that of glass, polycarbonate is used in optical applications such as eyeglass lenses, camera lenses, optical fibers, CD and DVD substrates, and fighter aircraft windows. Polycarbonate is the only colorless transparent material among general-purpose engineering plastics.
2. Impact Resistance
With the highest impact resistance of any plastic, polycarbonate is used in bulletproof materials, helmets, windshields, and more, with almost no risk of cracking when used in general environments.
3. Weather Resistance
Because it is resistant to ultraviolet rays and does not deteriorate easily, it maintains its high strength for a long period of time when used outdoors. It is used for outdoor items such as roofing materials and solar panel surface materials, as well as automobile headlights, roof rails, and door handles.
4. Dimensional Stability
Polycarbonate has high dimensional stability due to its low shrinkage and low moisture absorption during molding and is used for smartphone cases and other applications.
Characteristics of Polycarbonate
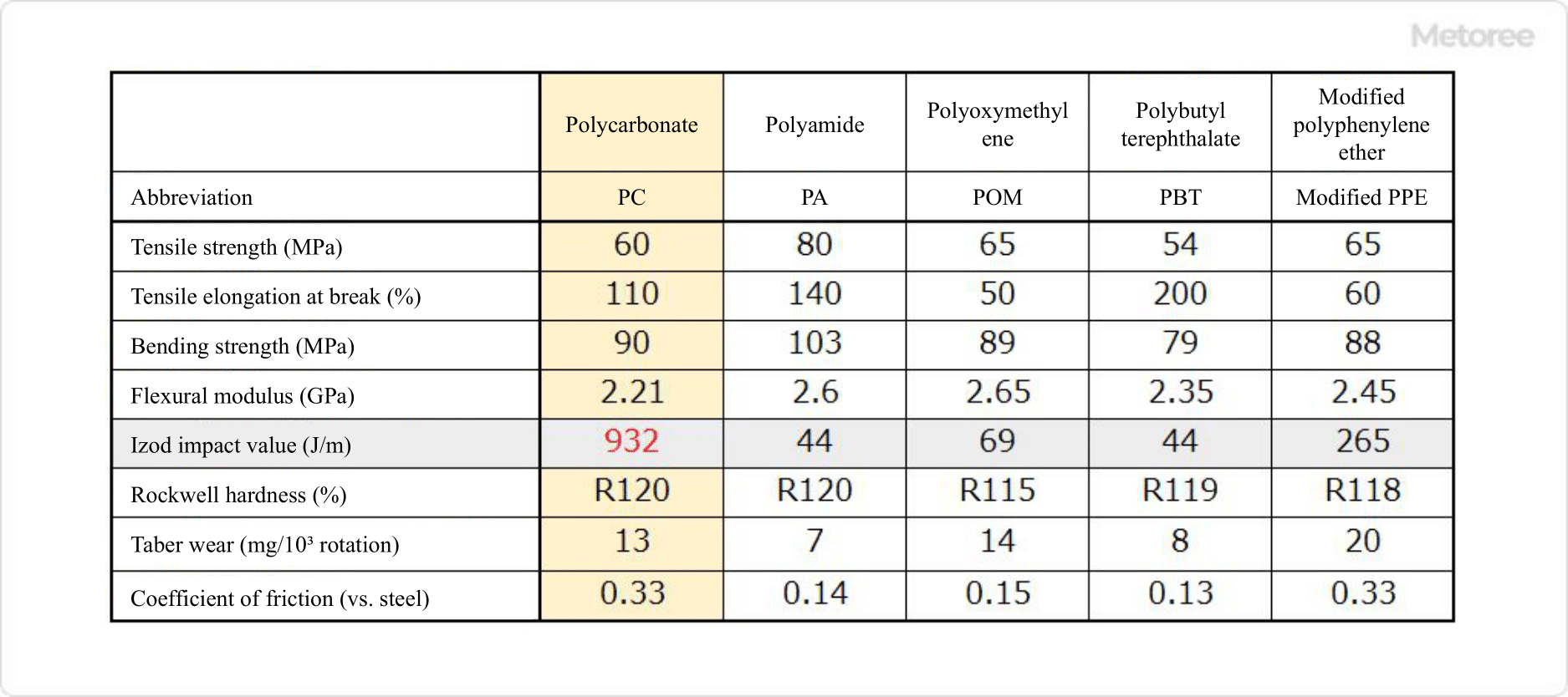
Figure 2. Five Major General-Purpose Engineering Plastics and Their Physical Properties (Refer to the 5th edition of the Chemical Handbook)
Polycarbonate is one of the five major general-purpose engineering plastics and is characterized by its high impact strength (20 times that of polyamide and polybutylene terephthalate) and resistance to cracking. In addition to its good mechanical strength, it is also used in various places because it has self-extinguishing properties (it does not spread even if it is ignited) since it contains two benzene rings in its main chain.
Polycarbonate is not used alone but is sometimes used in polymer alloys with other polymers. For example, PC-ABS is made by mixing Polycarbonate with ABS resin, which has good chemical resistance, to improve chemical resistance. In addition, polycarbonate can be blended with polyesters such as PET and PBT, or with fillers.
Another feature of Polycarbonate is that it can be used in a wide range of manufacturing processes. In addition to injection molding, polycarbonate can be extruded, vacuum molded, blow molded, and molded using a variety of other methods.
Recently, polycarbonate has also come to be used in 3D printers, which can easily produce complex shapes. However, depending on the manufacturing method, the mechanical strength may be weakened, so caution is required.
Polycarbonate Manufacturing Methods
Polycarbonate can be produced by two methods: polymerization using the reaction formula (interfacial polymerization) and ester exchange.
1. Interfacial Polymerization Method
Polycarbonate is produced by condensation polymerization at the interface between the aqueous and oil phases by adding carbonyl chloride to a suspension solution of bisphenol A in an aqueous sodium hydroxide solution and methylene chloride or chlorobenzene. Since the reaction conditions are milder than those of the transesterification method, polycarbonates with a wide range of molecular weights, from low to high, can be produced.
After polymerization, the Polycarbonate resin is dissolved in the oil phase, and granular Polycarbonate is obtained through a separation, neutralization, and purification process, as well as a polymer recovery process, and drying process.
2. Ester Exchange Method
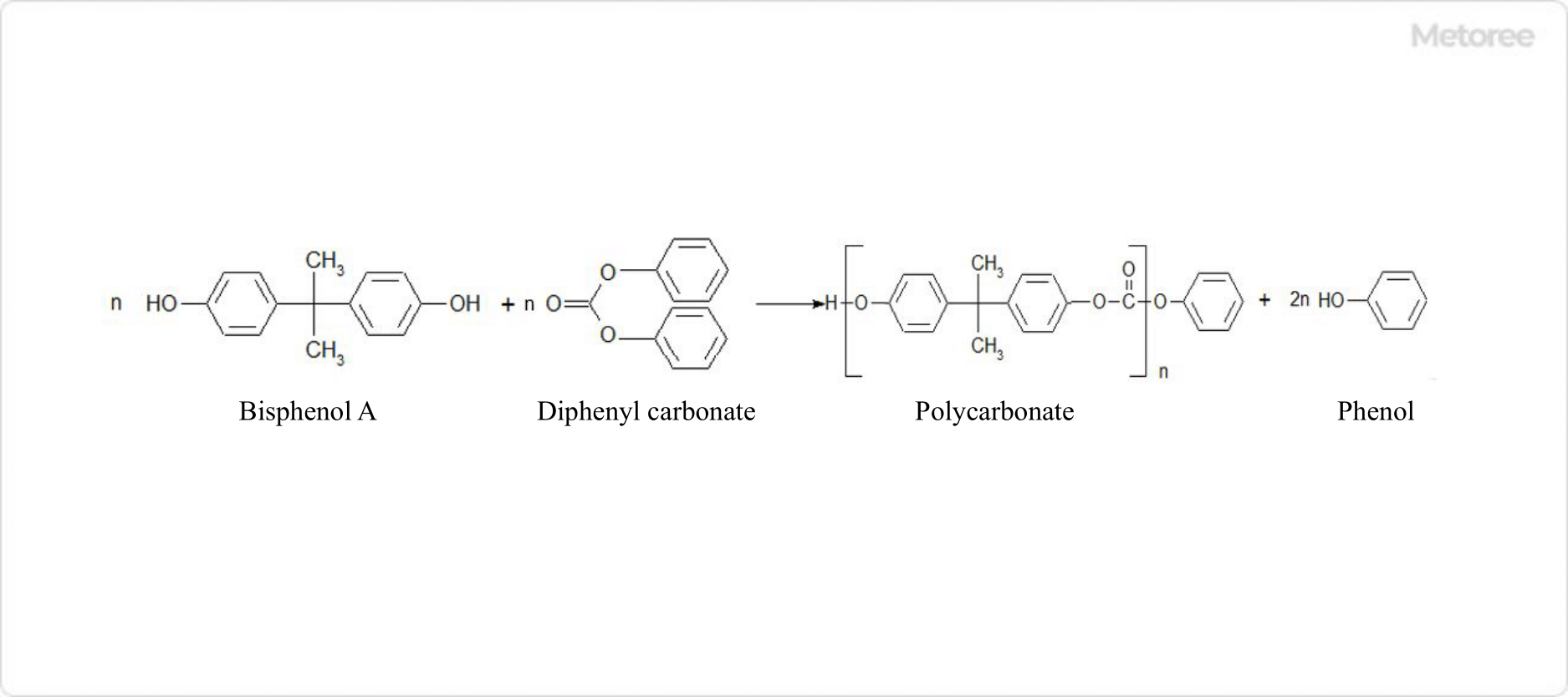
Figure 3. Ester Exchange Method Reaction Formula
Polycarbonate is produced by melting and mixing bisphenol A and diphenylcarbonate under the presence of a catalyst and polycondensing while recovering phenol under high temperature and reduced pressure without the use of solvents.
Polycarbonate is obtained in a pure molten state, not in solution, and can be pelletized into products, making this method a simpler synthesis process than the interfacial polymerization method.