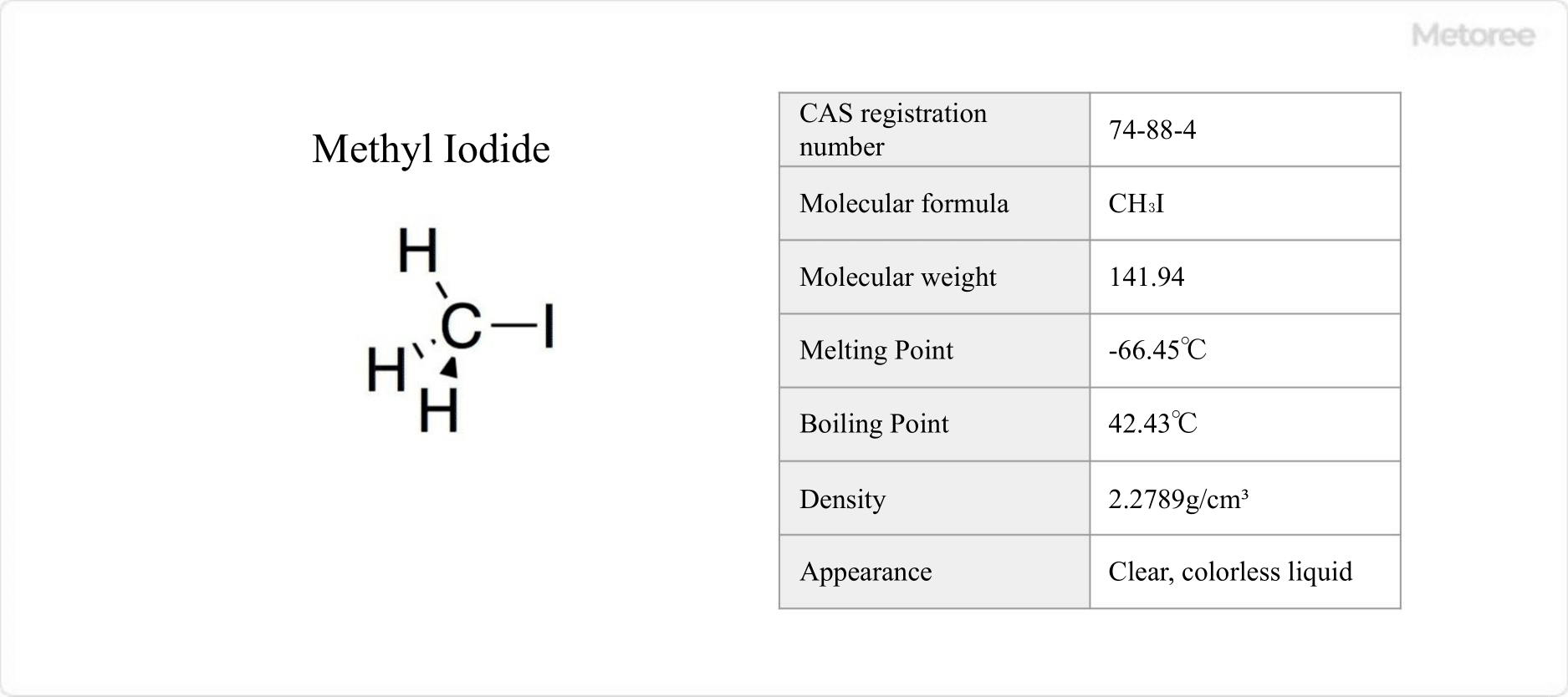
What Is Potassium Iodate?
Potassium iodate is a type of iodate salt with the chemical formula KIO3.
It is a colorless crystal that is soluble in water but insoluble in ethanol. It is stable at room temperature and decomposes into potassium iodide (KI) and oxygen when heated.
Potassium iodate is produced by reacting iodine with potassium hydroxide or by electrolyzing a potassium iodide solution. It is used in the coloring experiment of the “iodo-starch reaction” in chemistry, together with sodium thiosulfate and other substances.
Uses of Potassium Iodate
Potassium iodate is a strong oxidant and is used as an oxidant in chemical analysis, such as iodine reduction titration.
In other medical applications, it is used as a prophylactic for radiation damage and as a treatment for thyroid dysfunction due to iodine deficiency.
In overseas food products, it is added to powdered milk and salt as a dietary supplement to prevent iodine deficiency. In Japan, it is used as a feed additive to supplement nutritional components (iodine and potassium) in feed for industrial animals such as cattle, pigs, and chickens.