What Is Sodium Selenite?
Sodium selenite is an inorganic compound with the chemical formula Na2SeO3. It exists stably in both anhydrate and pentahydrate forms at room temperature and pressure, with CAS registration numbers 10102-18-8 (anhydrate) and 26970-82-1 (pentahydrate). This white powdery solid is naturally present in the bodies of animals.
Uses of Sodium Selenite
1. Industrial Applications
Sodium selenite serves various industrial purposes, including as a raw material for glass and pigments, a decolorizer for glass, an alkaloid reagent, and for surface treatment of light metals and coloring ceramics. It is also employed as a nutrient fortifier in the pharmaceutical and feed industries.
2. Clinical Uses
In clinical settings, sodium selenite is used in injectable dosage forms to treat hyposelenemia. This condition, characterized by selenium deficiency in the body, can lead to various symptoms, including nail whitening, muscle weakness, and myocardial damage. Sodium selenite injection solutions are crucial for addressing heart failure associated with selenium deficiency.
Properties of Sodium Selenite
Sodium selenite has a molecular weight of 172.94 and a melting point of 710°C (decomposition). It appears as a white crystalline powder at room temperature, with a density of 3.1 g/mL. While it is somewhat soluble in water (89.8 g/100 g), it is insoluble in ethanol.
Types of Sodium Selenite
Sodium selenite is available in different forms for various applications:
1. Reagent Products for Research and Development
Reagent-grade sodium selenite comes in various capacities, from 1g to 500g, and is used for research purposes. Proper storage below 25°C is necessary.
2. Pharmaceutical Products
Sodium selenite is sold in injectable solutions for clinical use in treating hyposelenemia.
3. Industrial Raw Materials
For industrial applications, sodium selenite is provided in bulk quantities, such as 25 kg fiber drums, for ease of handling in factories.
Other Information on Sodium Selenite
1. Synthesis
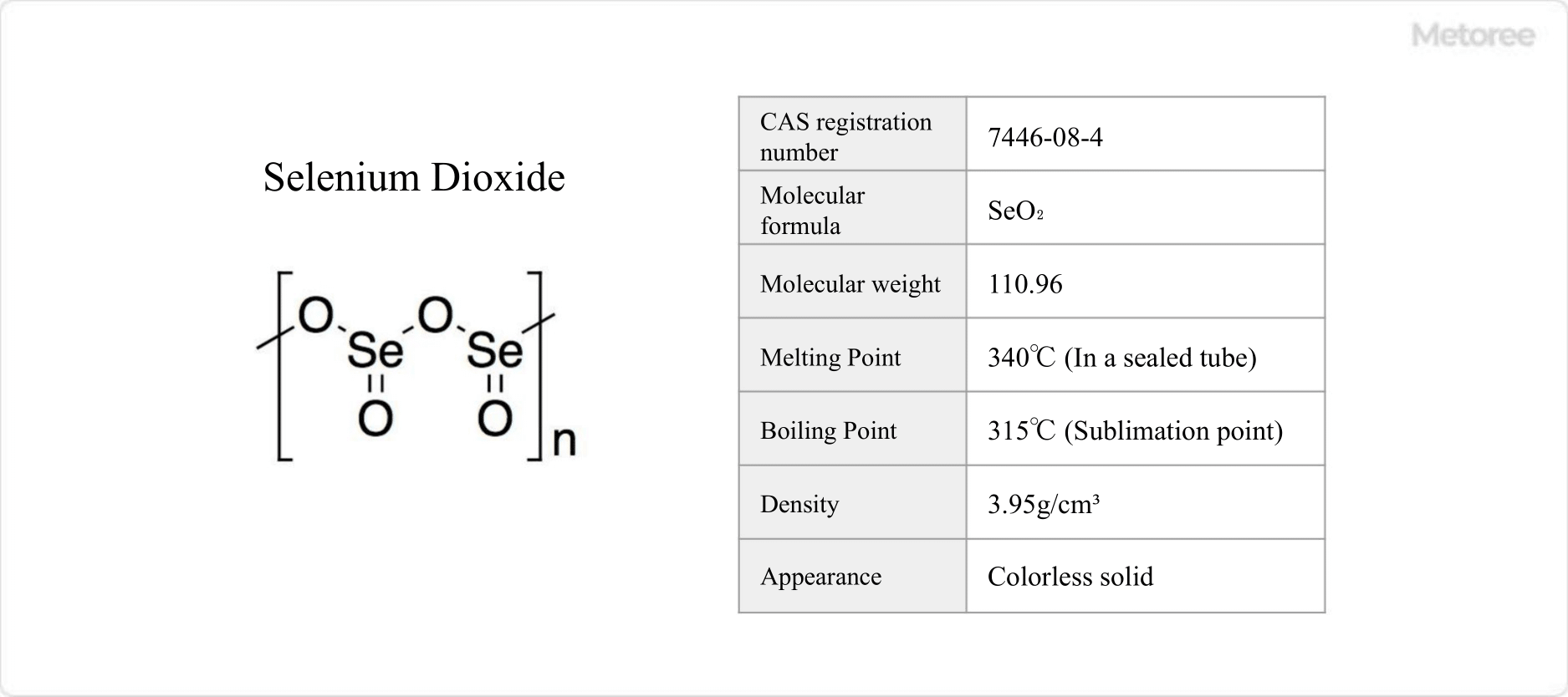
Sodium selenite is synthesized by reacting selenium dioxide with sodium hydroxide, with the hydrate losing its hydration water at 40°C to become anhydrous.
2. Hazardous Properties and Regulations
Sodium selenite is harmful to human health and is regulated under various laws. It is important to handle it in compliance with legal requirements due to its toxicity and potential health hazards.