What Is Selenium Dioxide?
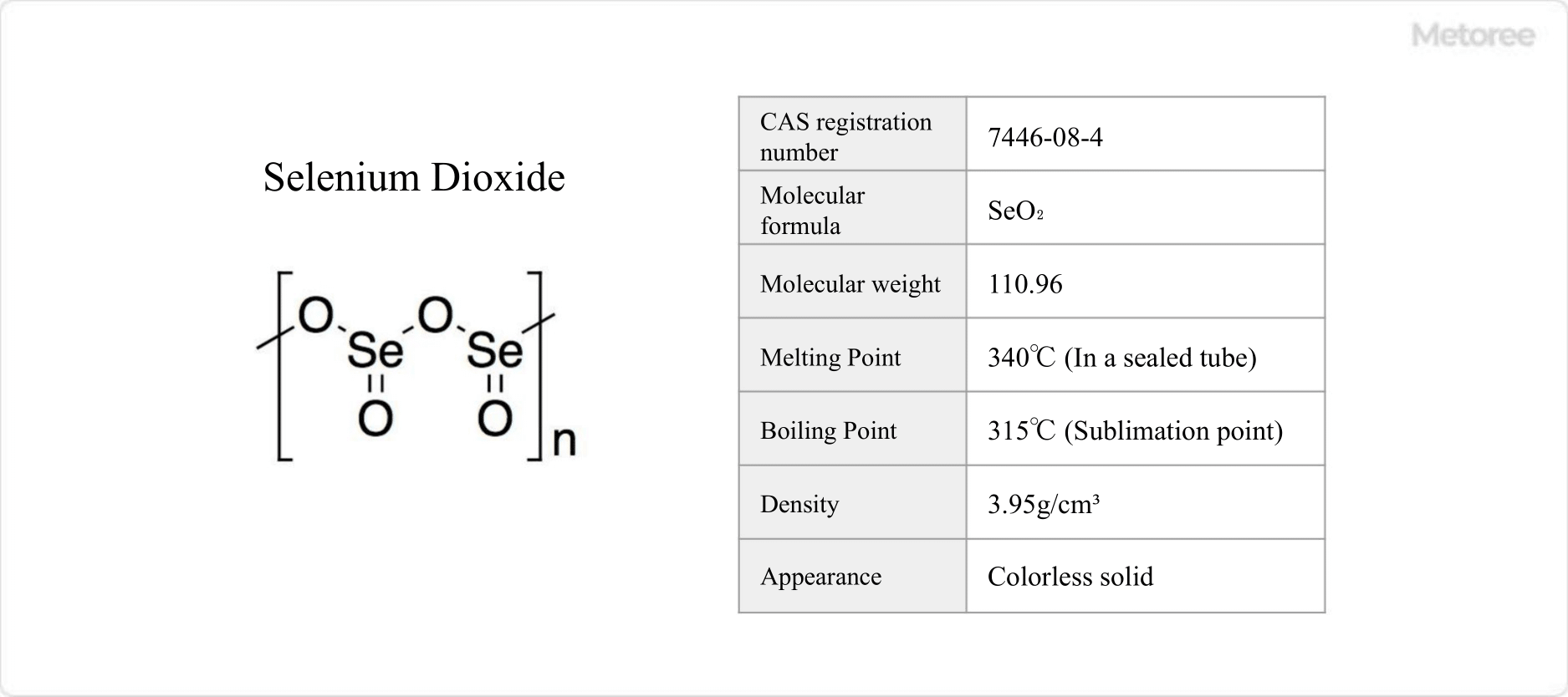
Figure 1. Basic Information on Selenium Dioxide
Selenium dioxide, also known as selenous anhydride, is an oxide of selenium formed by burning selenium or oxidizing it with nitric acid. It appears as a colorless crystal at room temperature.
Under the Industrial Safety and Health Law, selenium dioxide is classified as a hazardous and toxic substance. It is also recognized as a chemical hazard under the Labor Standards Law, a class 1 designated chemical substance under the PRTR Law, and a toxic substance under the Poisonous and Deleterious Substances Control Law.
Uses of Selenium Dioxide
Selenium dioxide serves as an oxidizing agent in organic chemistry, enabling the synthesis of compounds that are challenging to produce by other means. It is particularly useful for oxidizing carbonyl compounds, olefins, acetylenes, and alcohols. Additionally, it is used in glassmaking to decolorize glass with impurities, giving it a colorless appearance.
Properties of Selenium Dioxide
At room temperature, selenium dioxide forms colorless, needle-like crystals. It sublimates at 315°C into a yellowish-green gas and melts at 340°C in a sealed tube, turning into a blue liquid. It dissolves in water at 73.3 g per 100 g at 25°C, emits a characteristic unpleasant odor, and is highly toxic. The LD50 values are 23.3 mg/kg for mice and 68.1 mg/kg for rats when administered orally. It can be absorbed through inhalation and skin contact, necessitating careful handling.
Structure of Selenium Dioxide
Selenium dioxide has a chemical formula of SeO2, a formula weight of 110.96 g/mol, and a density of 3.95 g/cm3. It forms chain-like polymers at room temperature, with alternating selenium and oxygen atoms. The polymer’s selenium atoms are pyramidal, with oxide groups at the ends. In the gas phase, it exists as dimers and oligomers, and at high temperatures, it becomes monomeric, adopting a curved structure similar to that of sulfur dioxide.
Other Information on Selenium Dioxide
1. Reaction of Selenium Dioxide
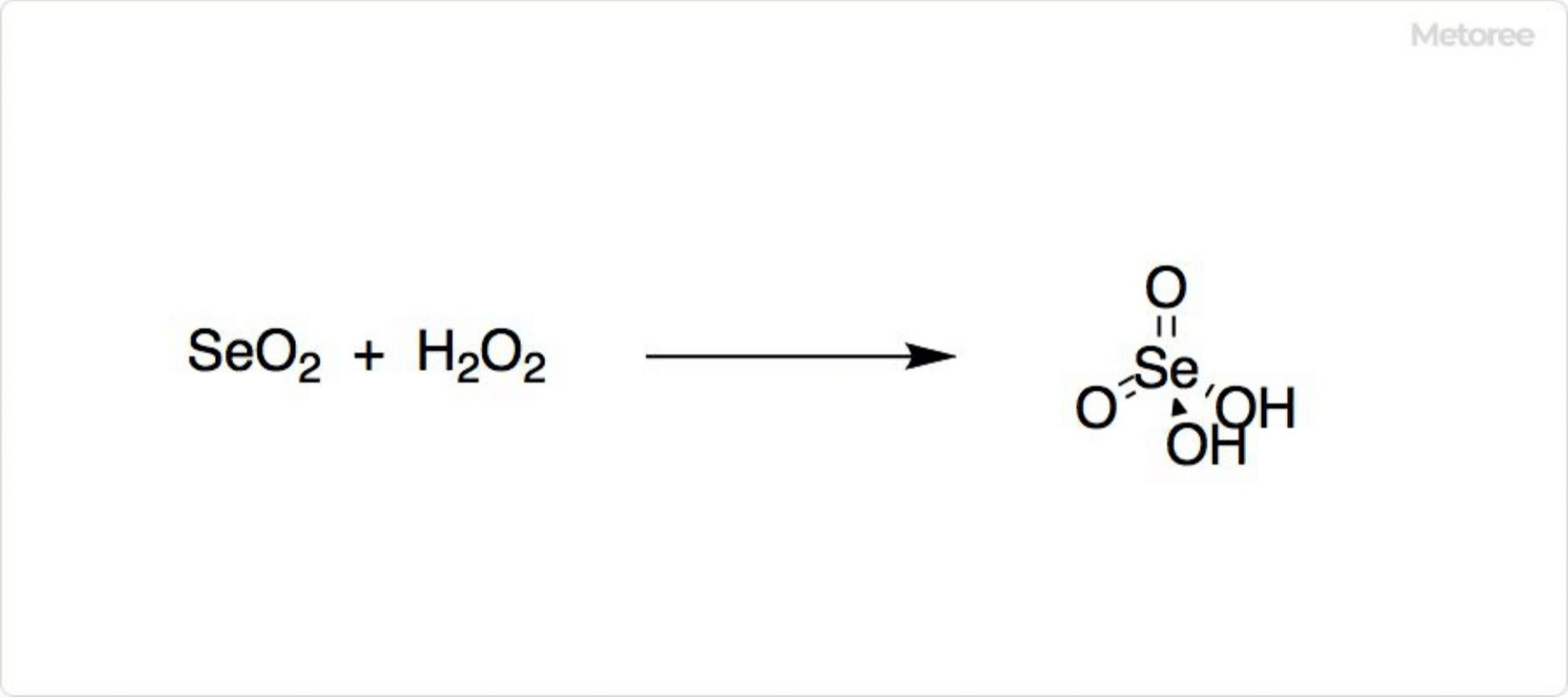
Figure 2. Reactions of Selenium Dioxide
When reduced by ammonia, selenium dioxide forms elemental selenium. Oxidation by hydrogen peroxide or oxygen in nitric acid yields selenite.
2. Reaction With Selenium Dioxide
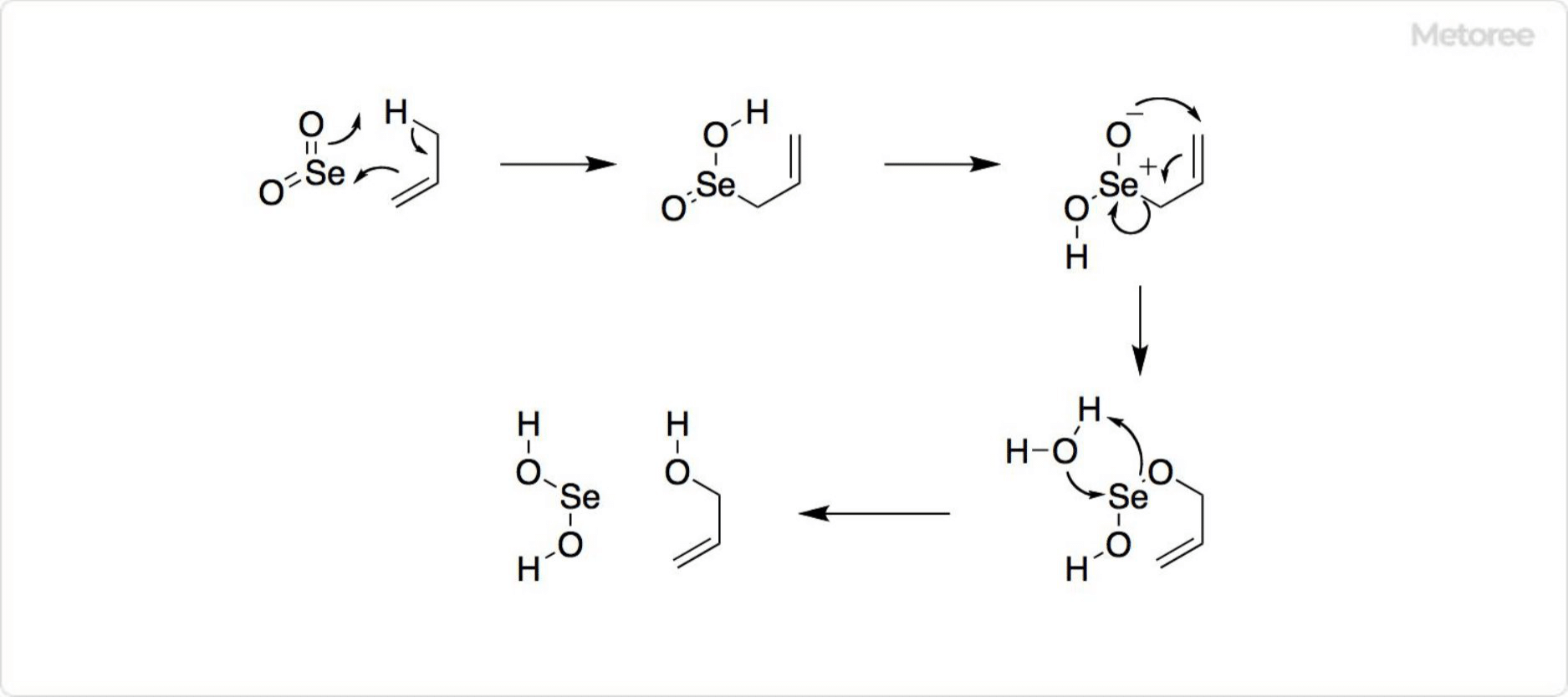
Figure 3. Reaction using Selenium Dioxide
As a valuable reagent in organic synthesis, selenium dioxide can oxidize various organic compounds, such as converting paraldehyde to glyoxal and cyclohexanone to 1,2-cyclohexanedione. The oxidation reactions, including Riley oxidation, highlight its utility in synthesizing complex molecules like 1,2,3-selenadiazoles from acylated hydrazone derivatives.