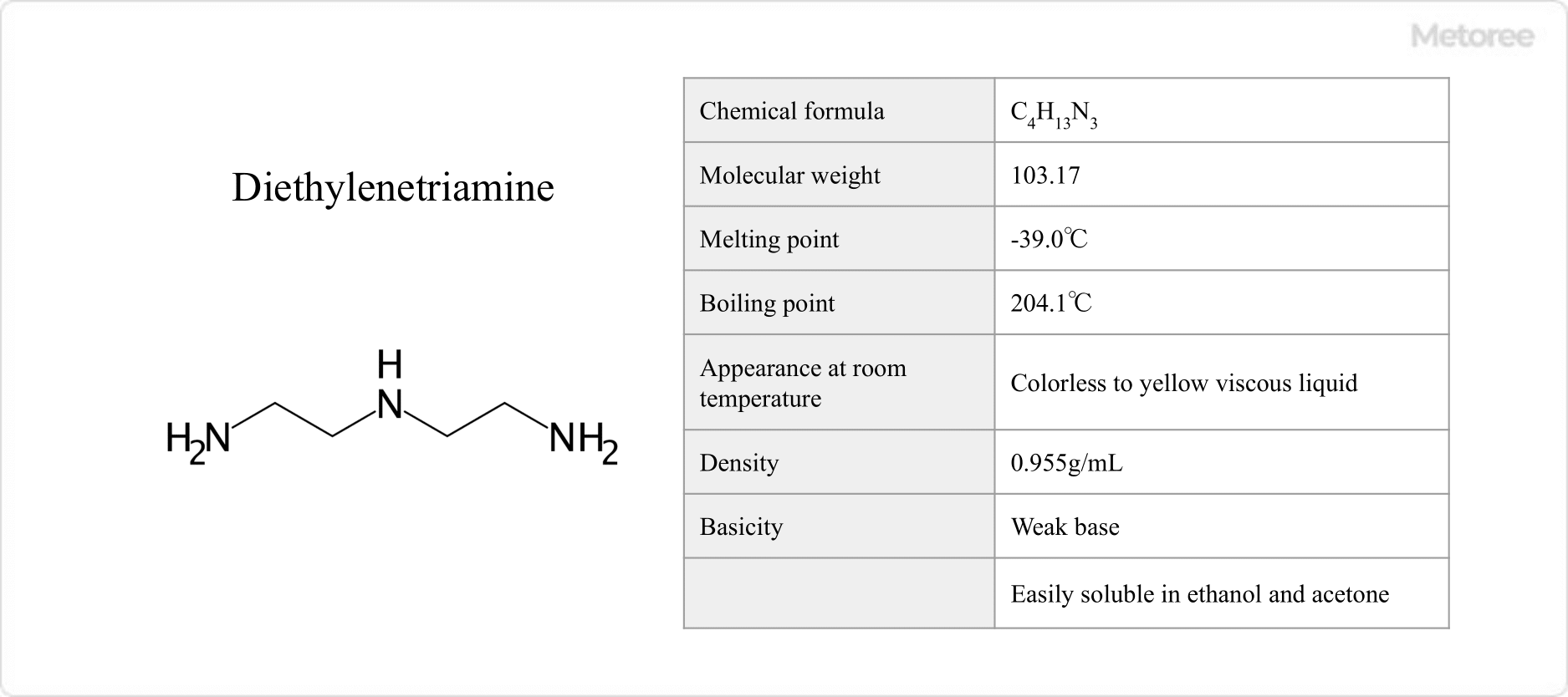
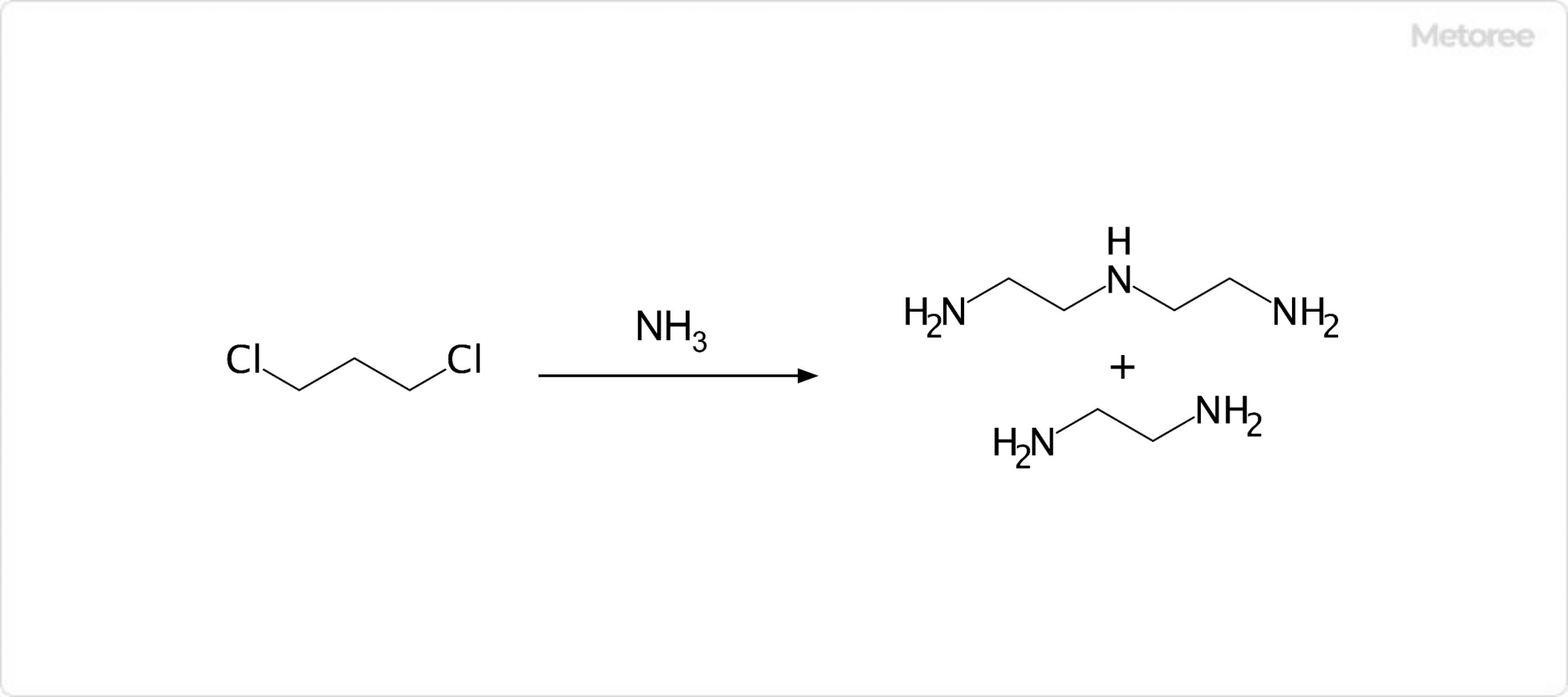
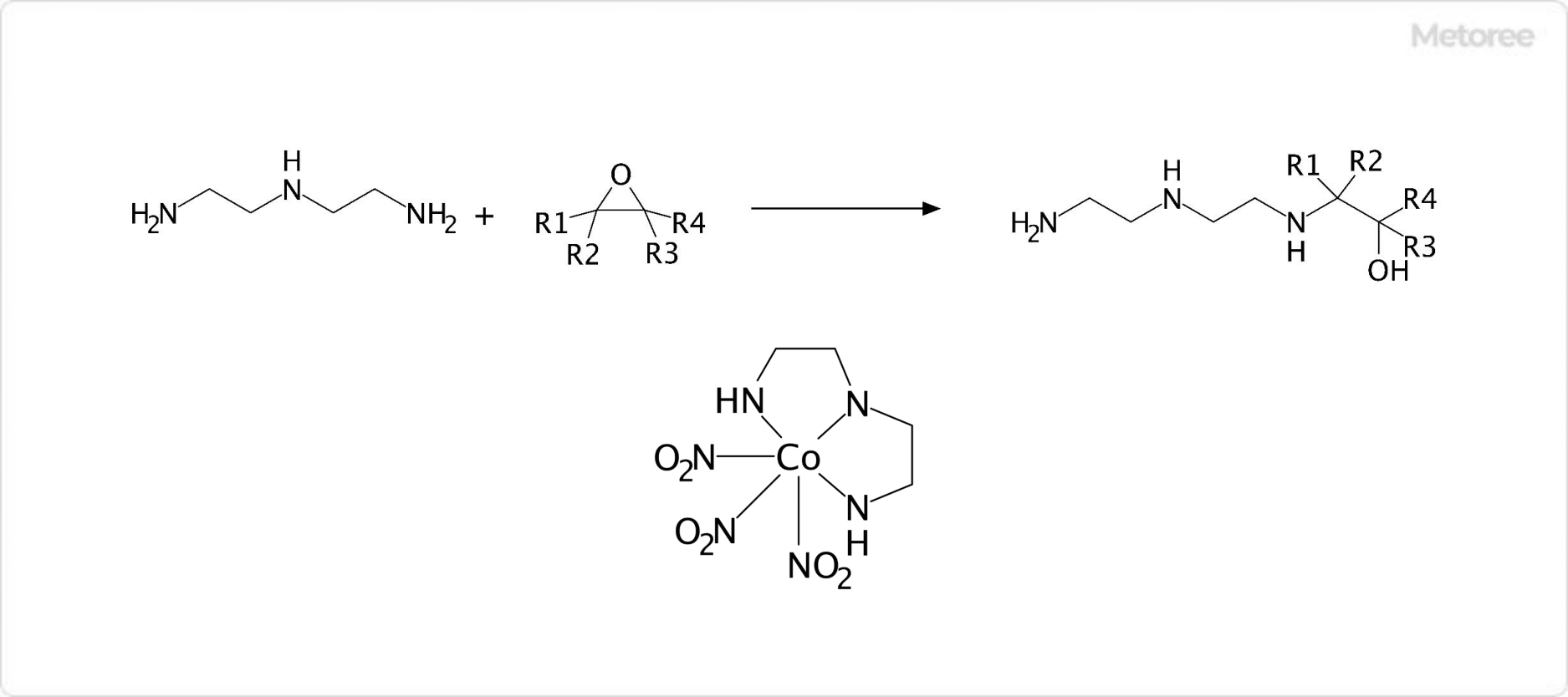
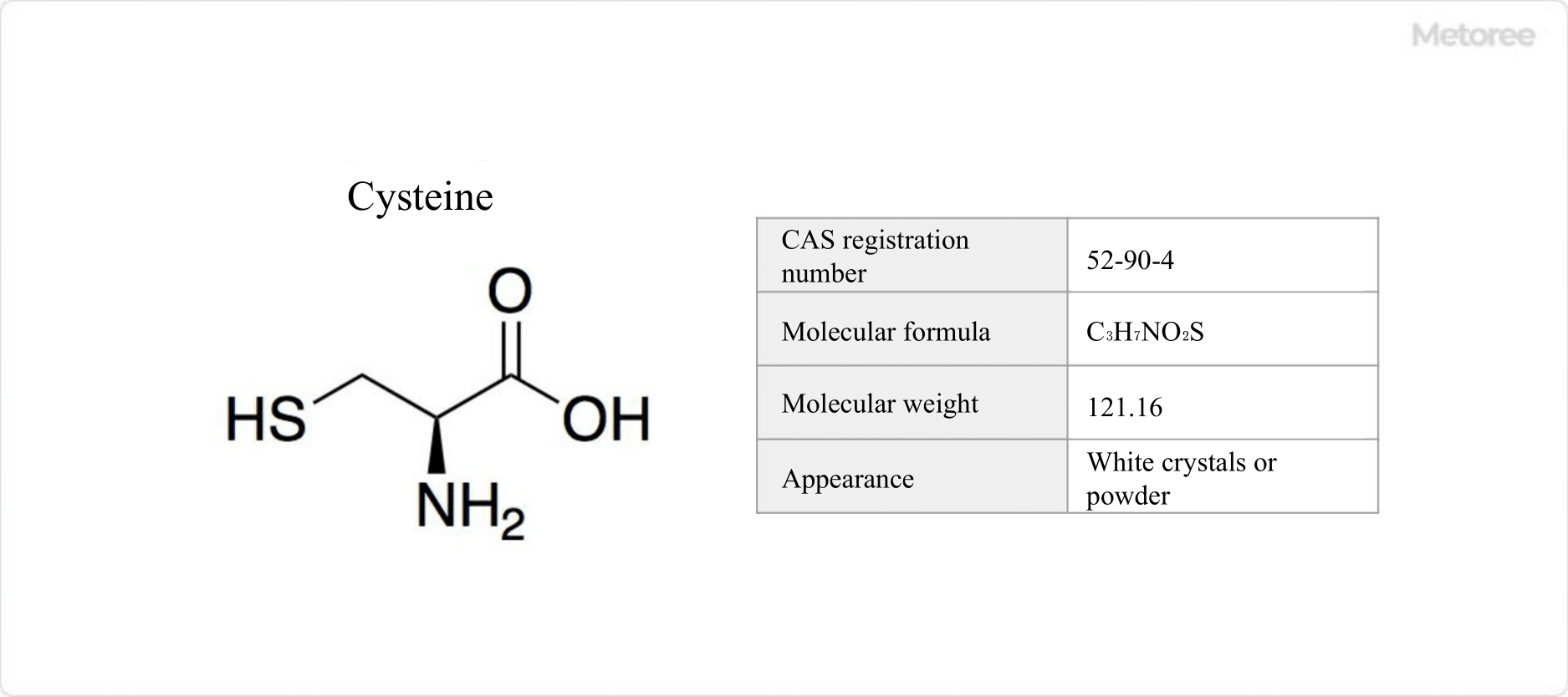
What Is Strontium?
Strontium, symbol Sr, is an alkaline earth metal with an atomic number 38 and an atomic weight of 87.62. This silvery-white metallic element is highly reactive, violently reacting with water and tarnishing in air. Its salts are used to produce red colors in fireworks due to their striking red flame coloration.
Uses of Strontium
Strontium’s primary applications include serving as an additive for display and photovoltaic glass, manufacturing ferrite magnets, ceramic capacitors, and rust inhibitors. It also plays a role in producing red colors for fireworks and smoke bombs through its nitrates.
Properties of Strontium
Similar to calcium in its physiological behavior, strontium has a melting point of 777°C and boils at 1,382°C. It forms a grayish-white oxide layer when exposed to air and reacts explosively with water. Strontium can be obtained by reducing its oxide with aluminum or by electrolysis of strontium chloride.
Structure of Strontium
At room temperature, strontium adopts a face-centered cubic lattice structure. It transitions to hexagonal close-packed from 213°C to 621°C and to body-centered cubic from 621°C to 769°C. Its electron configuration is [Kr] 5s2, allowing it to substitute calcium in minerals.
Other Information on Strontium
1. Production of Strontium
China, Spain, and Mexico lead in strontium production, primarily extracting it from the mineral celestite (SrSO4). The extraction process often involves converting celestite to strontium carbonate, which is then utilized in various applications.
2. Isotopes of Strontium
Natural strontium consists of four stable isotopes, with 88Sr being the most abundant. The radioactive 90Sr, with a half-life of 28.78 years, is noteworthy for its use in radioisotope thermoelectric generators. Strontium isotopes have significant applications in geology and nuclear science.