What Is L-Cysteine?
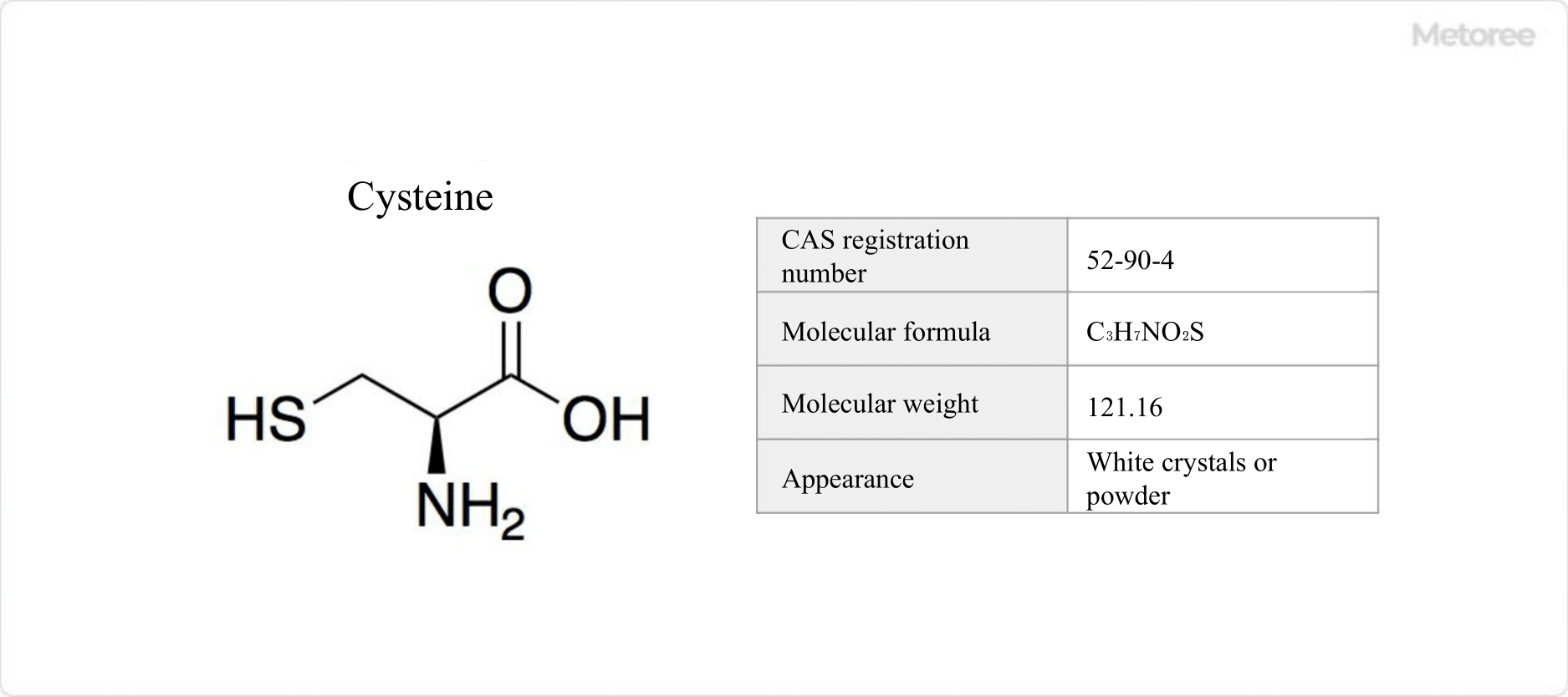
Figure 1. Basic Information on L-Cysteine
L-cysteine is a nonessential amino acid characterized by a thiol group on its side chain.
Its abbreviation is Cys, and L-cysteine is the naturally occurring form. It plays a role in reducing the production of melanin, which causes skin pigmentation, and promotes its elimination from the body.
L-cysteine, being a constituent of proteins, reacts with acetaldehyde, a primary cause of hangovers, neutralizing its toxicity. Its effectiveness in mitigating hangovers has led to its use in health supplements.
Uses of L-Cysteine
L-cysteine is used in pharmaceuticals and as a food additive. It exhibits antioxidant properties in the body and aids in liver detoxification.
Its applications include use in anti-allergic medications, treatments for skin conditions, and as a countermeasure against leukopenia resulting from radiation exposure. It is also valued in cosmetic supplements for its skin-lightening effects when combined with vitamin C.
Additionally, it is popularly employed as a remedy for hangovers.
Properties of L-Cysteine
L-cysteine, a protein-building amino acid, is glycogenic. It exhibits specific optical rotation [α]D of +9.4° and decomposes at 240°C.
The thiol group in L-cysteine serves as a nucleophilic catalyst and displays high reactivity. The pKa of this group is around 8, varying with environmental conditions.
Structure of L-Cysteine
Figure 2. Structure of L-Cysteine
L-cysteine is 2-amino-3-sulfanyl propionic acid, considered hydrophobic or having a neutral polar side chain.
Due to its chiral carbon atom, it exists as R-cysteine (L-cysteine) and S-cysteine (D-cysteine). By the rank order rule, L-cysteine uniquely assumes the R configuration. Its chemical formula is C3H7NO2S with a molar mass of 121.16 g/mol.
Other Information on L-Cysteine
1. Biosynthesis of L-Cysteine
Biosynthesis in animals begins with serine, converting methionine to homocysteine, then to cystathionine via cystathionine β-synthetase, and finally to L-cysteine.
In plants and bacteria, L-cysteine is synthesized from serine through O-acetylserine, with cysteine synthase facilitating the final step.
2. Oxidation of L-Cysteine
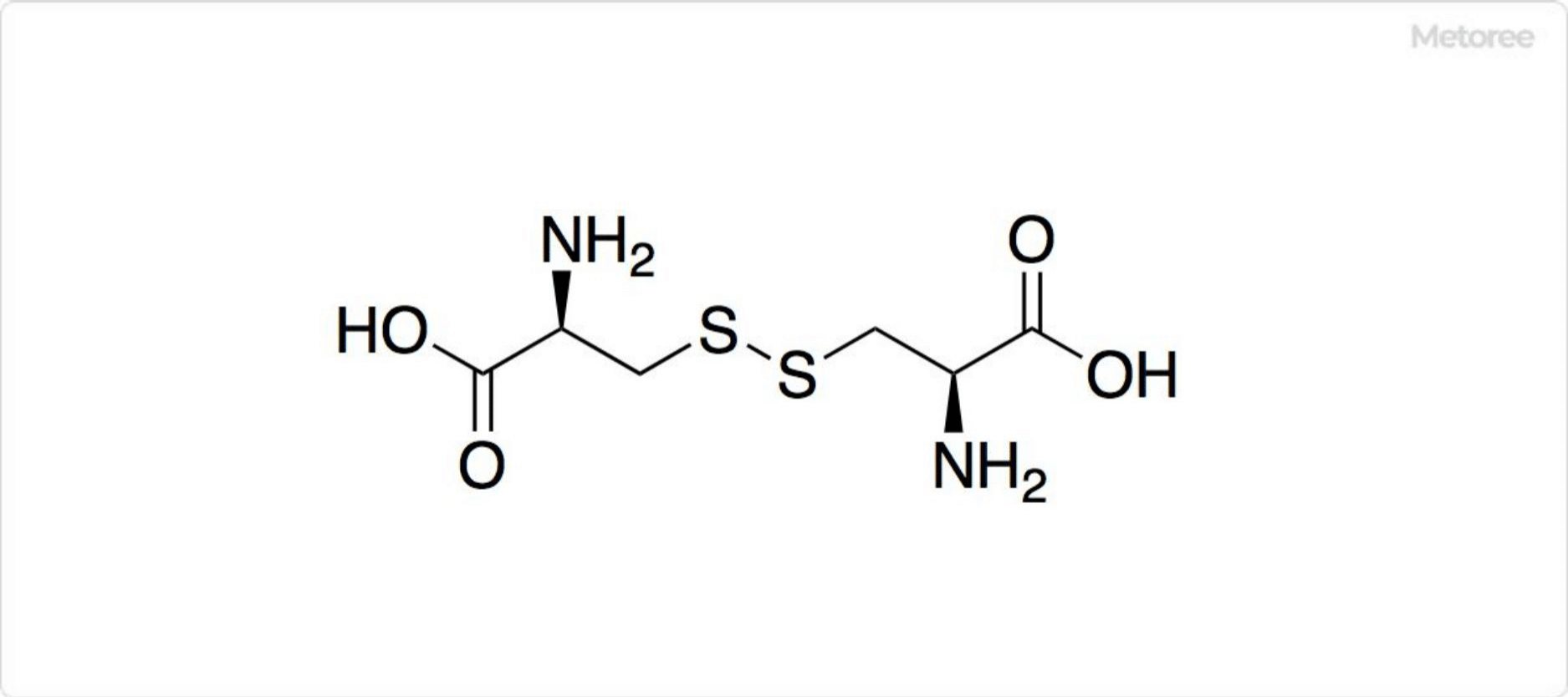
Figure 3. Disulfide Bonds in L-Cysteine
L-cysteine forms cystine upon oxidation, which involves the linkage of two L-cysteine molecules via a disulfide bond.
This oxidized form, referred to as cystine, loses its nucleophilic properties.
3. Biochemistry of L-Cysteine
L-cysteine’s thiol group facilitates protein cross-linking via disulfide bonds, crucial for the stability and functional integrity of proteins both inside and outside the cell.
For instance, insulin’s structure and function are dependent on disulfide bonds between L-cysteine residues, demonstrating its significance in biochemistry.