What Is Thiourea?
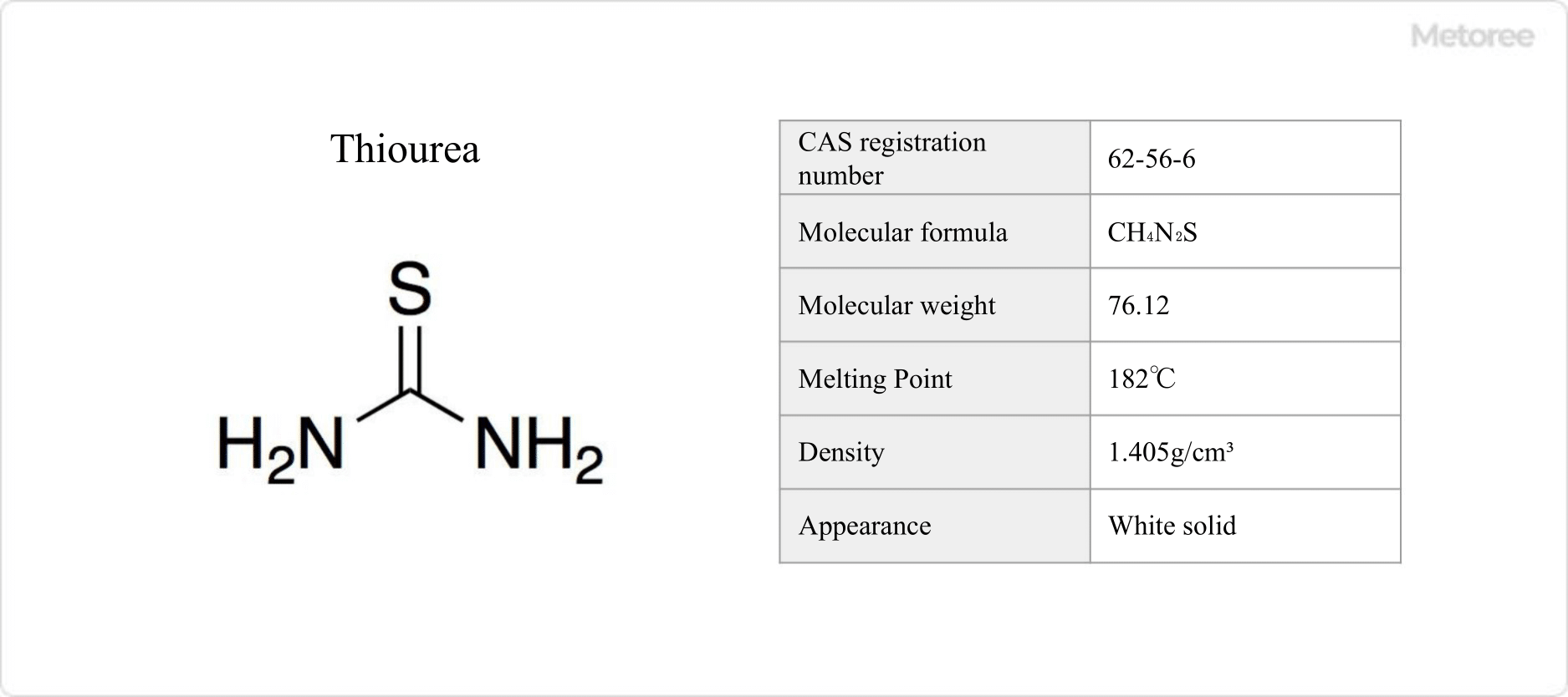
Figure 1. Basic Information on Thiourea
Thiourea, an organosulfur compound replacing urea’s oxygen with sulfur, is utilized in various industries for its unique chemical properties and applications, from pharmaceuticals to dyes and rubber additives.
Uses of Thiourea
Key uses span urethane resins a polymer with urethane bonds, also called polyurethane or urethane rubber production, as well as pharmaceuticals, dyes, and as a source of hydrogen sulfide in qualitative analysis, showcasing its broad utility.
Properties of Thiourea
Soluble in water and ethanol, thiourea is known for its planar structure, facilitating its role in redox reactions and complex formation with metals.
Structure of Thiourea
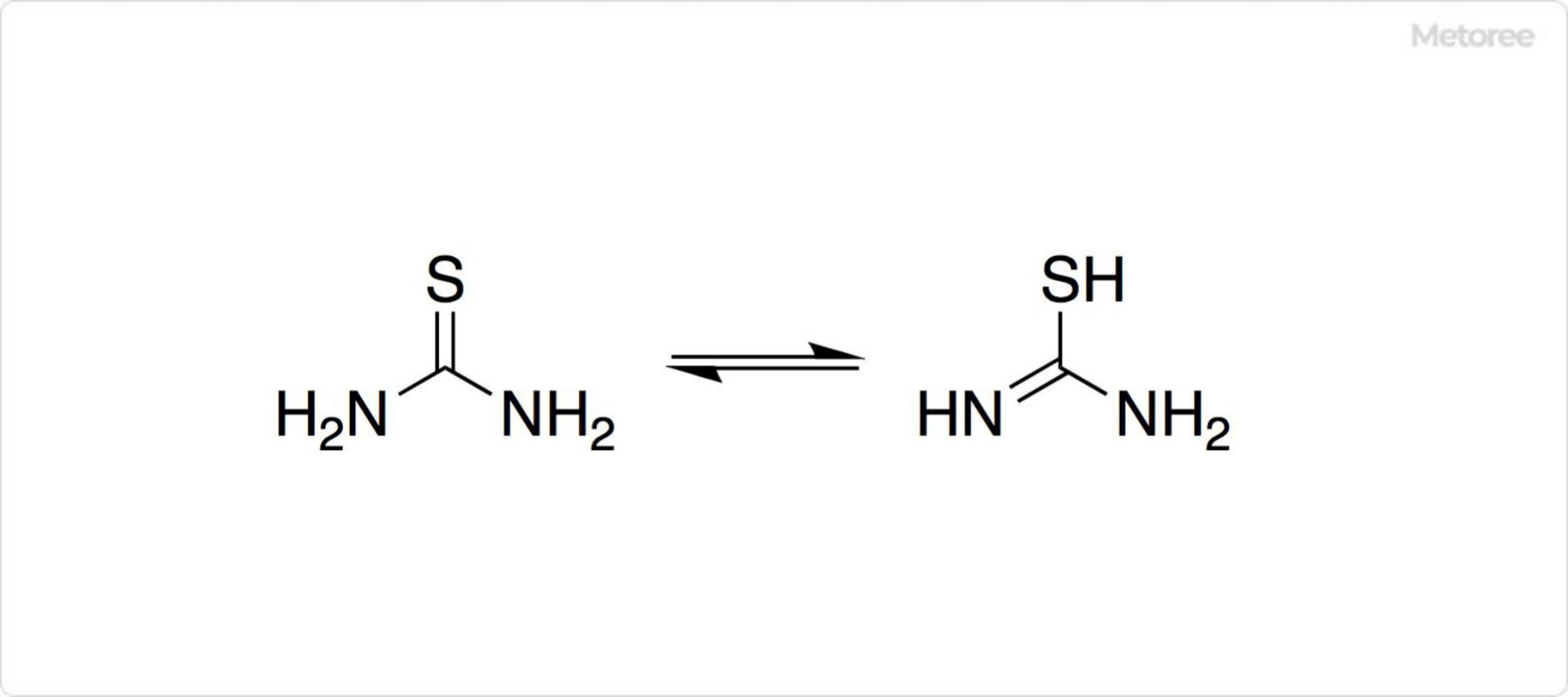
Figure 2. Structure of Thiourea
The molecule’s structure, featuring both thiol and amine functionalities, underpins its reactivity and applications in catalysis and organic synthesis.
Other Information on Thiourea
1. Reduction Reaction Using Thiourea
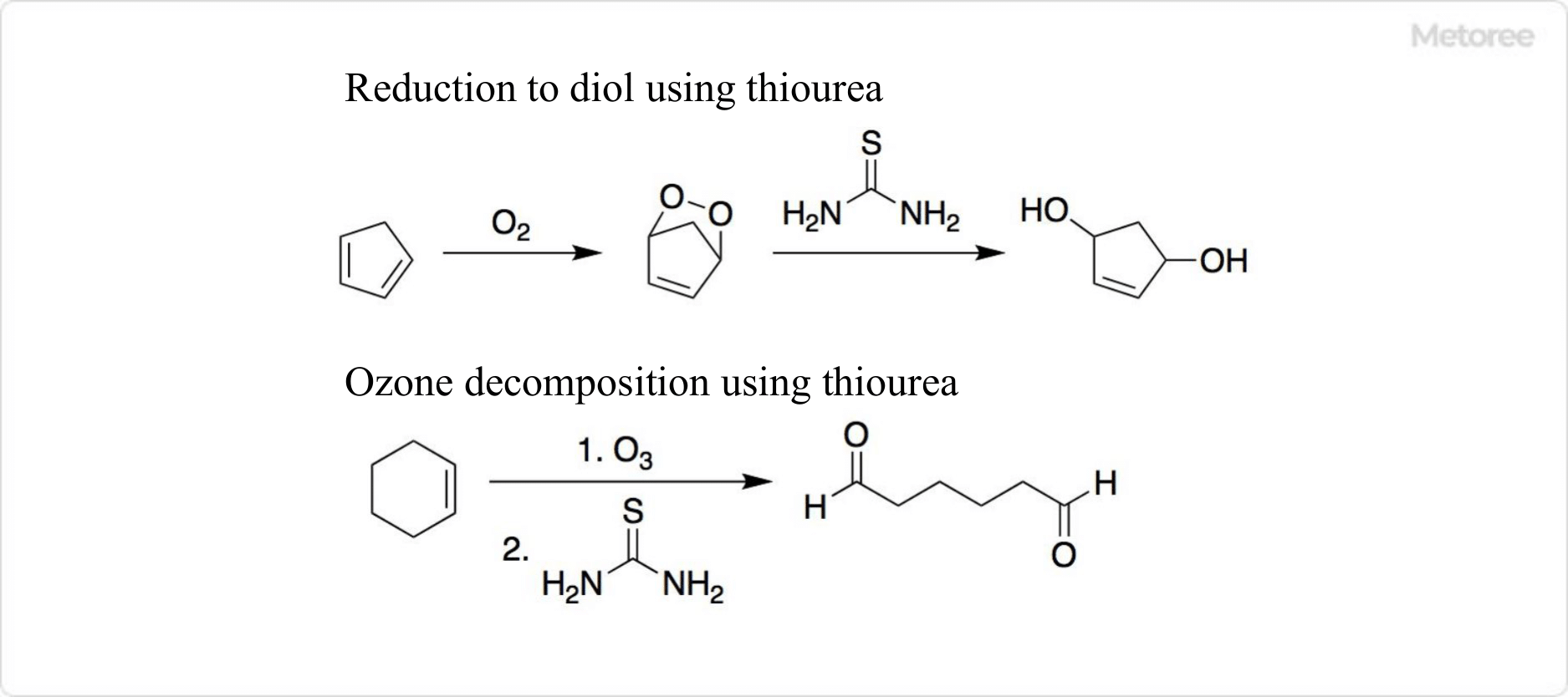
Figure 3. Reaction Using Thiourea
Thiourea’s capability to reduce peroxides to diols and serve as a reducing agent in ozone decomposition is highlighted, alongside its odorless and less volatile nature compared to other sulfur sources.
2. Thiourea as a Sulfur Source
Its function as a sulfur atom source in synthetic chemistry, particularly in the synthesis of metal sulfides and organic thiols, underlines its versatility and importance in material science and organic synthesis.