What Is a Dispersant?
A dispersant is an agent used to uniformly disperse particles in a medium and maintain a stable dispersion state without re-agglomeration.
Dispersants can be broadly classified into two types: surfactant-type dispersants and polymer-type dispersants. Surfactant-type dispersants consist of hydrophilic and hydrophobic groups and are categorized as anionic, cationic, or nonionic based on the component of the hydrophilic group.
Principle of Dispersants
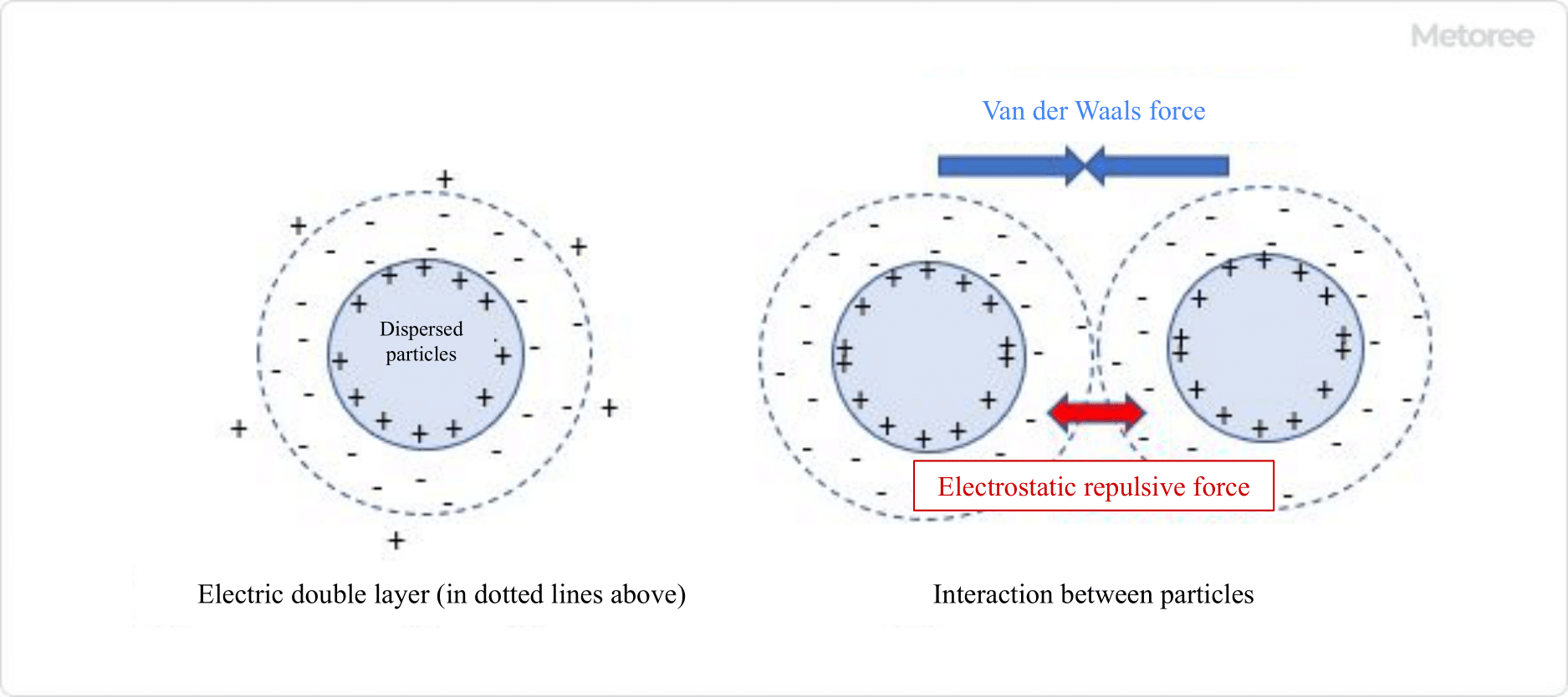
Figure 1. Interaction between the electric double layer and particles
Dispersants achieve dispersion by two mechanisms: electrostatic repulsion and steric hindrance repulsion. These mechanisms prevent particles from agglomerating in a liquid. The balance between electrostatic repulsion and cohesive forces, such as Van der Waals forces, determines whether particles will aggregate or disperse. When Van der Waals forces dominate, particles tend to aggregate and settle.
1. Electrostatic Repulsion
Particles in a dispersant carry charges and ions with opposite charges surround the particles, forming an electric double layer. Thickening this electric double layer with the help of dispersants increases the repulsive force between particles, preventing agglomeration.
2. Steric Hindrance Repulsion
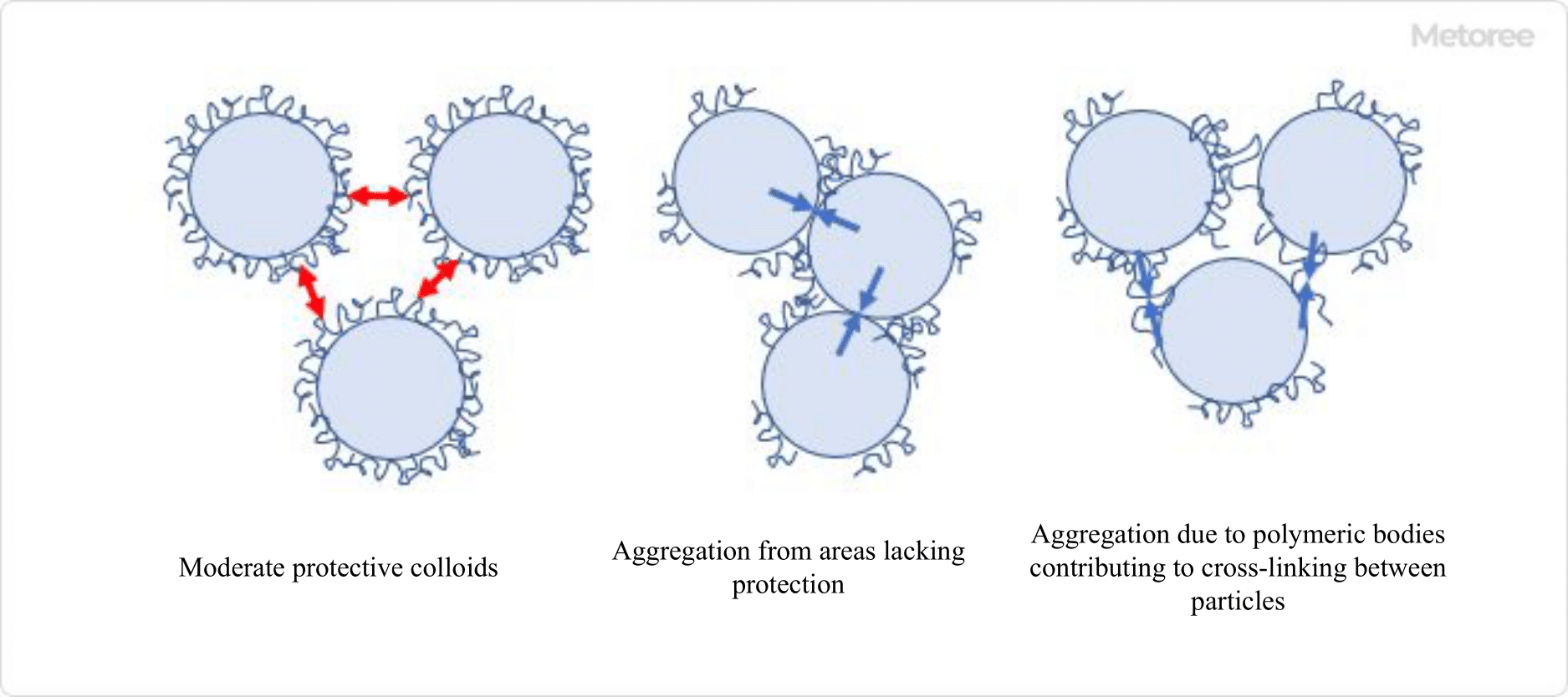
Figure 2. Protective colloidal effect of polymeric dispersants
Polymeric dispersants with large molecular weights adsorb to particle surfaces, creating a protective colloidal layer that hinders particle aggregation. Increasing the number of molecules in this layer leads to a bulkier structure, making it difficult for particles to come into close contact and promoting dispersion stabilization.
When organic solvents are used as dispersing solvents, electrostatic repulsion is weaker than in aqueous systems. Therefore, dispersion via steric hindrance repulsion is employed. When selecting a polymer-based dispersant, both the molecular structure and molecular weight are crucial. Higher molecular weights offer stronger protective colloidal effects, but if the molecular weight exceeds a certain point, the dispersant molecules can absorb two or more particles, leading to increased agglomeration. Thus, selecting an appropriate molecular weight is essential.
Types of Dispersants
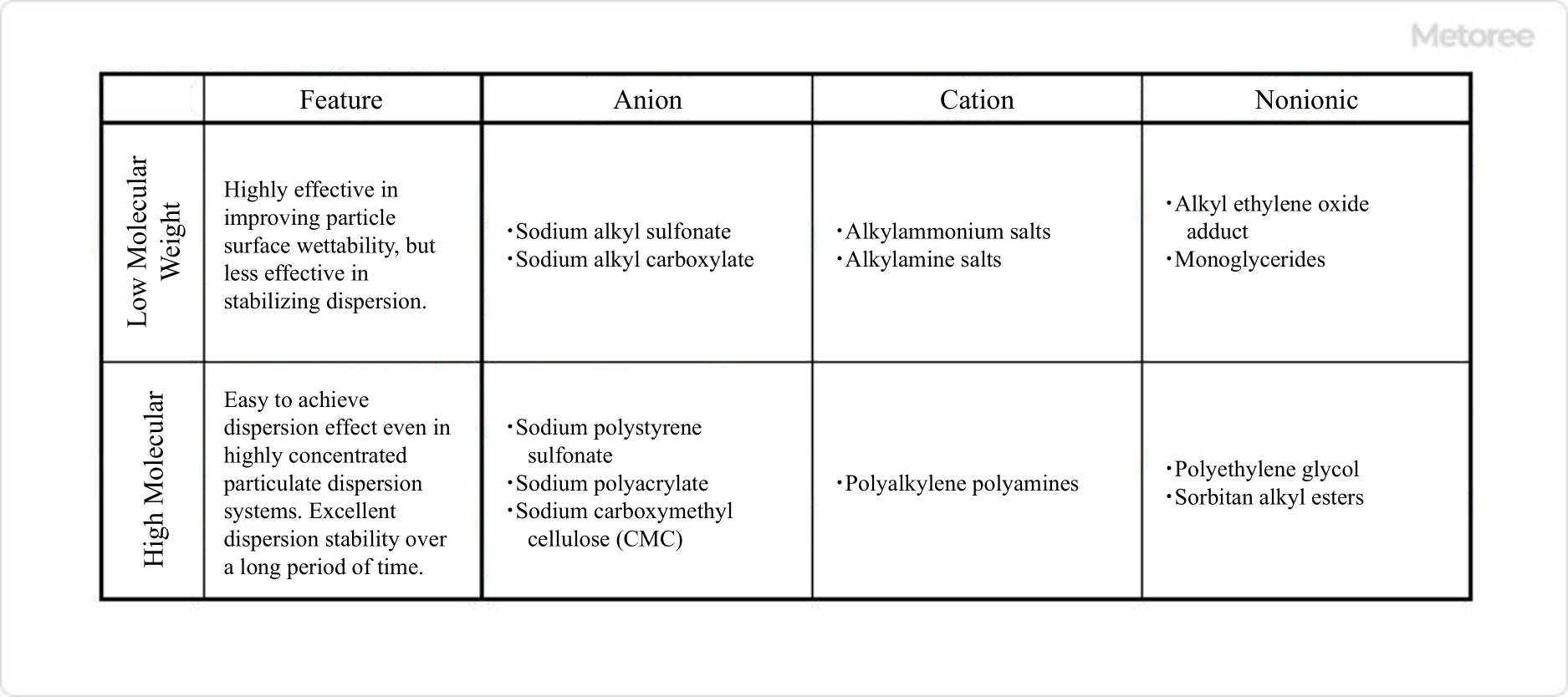
Figure 3. Types and characteristics of dispersants
Dispersant types include surfactant-type dispersants, polymer-type dispersants, and inorganic-type dispersants. Among them, surfactant-type dispersants can be further classified as anionic, cationic, or nonionic.
How to Select Dispersants
The choice of dispersant depends on the quality of the dispersant, the dispersant medium, and the dispersant concentration. However, when dispersing in water, the following three points are crucial:
- Select a dispersant that dissolves well in water and can be easily absorbed.
- For smaller particle sizes, use a surfactant-type dispersant with excellent wetting properties to reduce interfacial energy and minimize cohesive forces.
- For high dispersion concentrations, polymer-type dispersants that provide steric hindrance repulsion are effective.
Other Information on Dispersants
Functions of Dispersants Beyond Dispersion
Dispersants also serve functions beyond dispersion, such as improving the wettability of base materials. In applications like paints and other coatings, poor wettability can lead to paint flaking. This issue is caused by surface tension, which minimizes contact between the liquid and the coated object. Adding dispersants reduces surface tension, facilitating paint spreading on the coated surface.