What Is Valeric Acid?
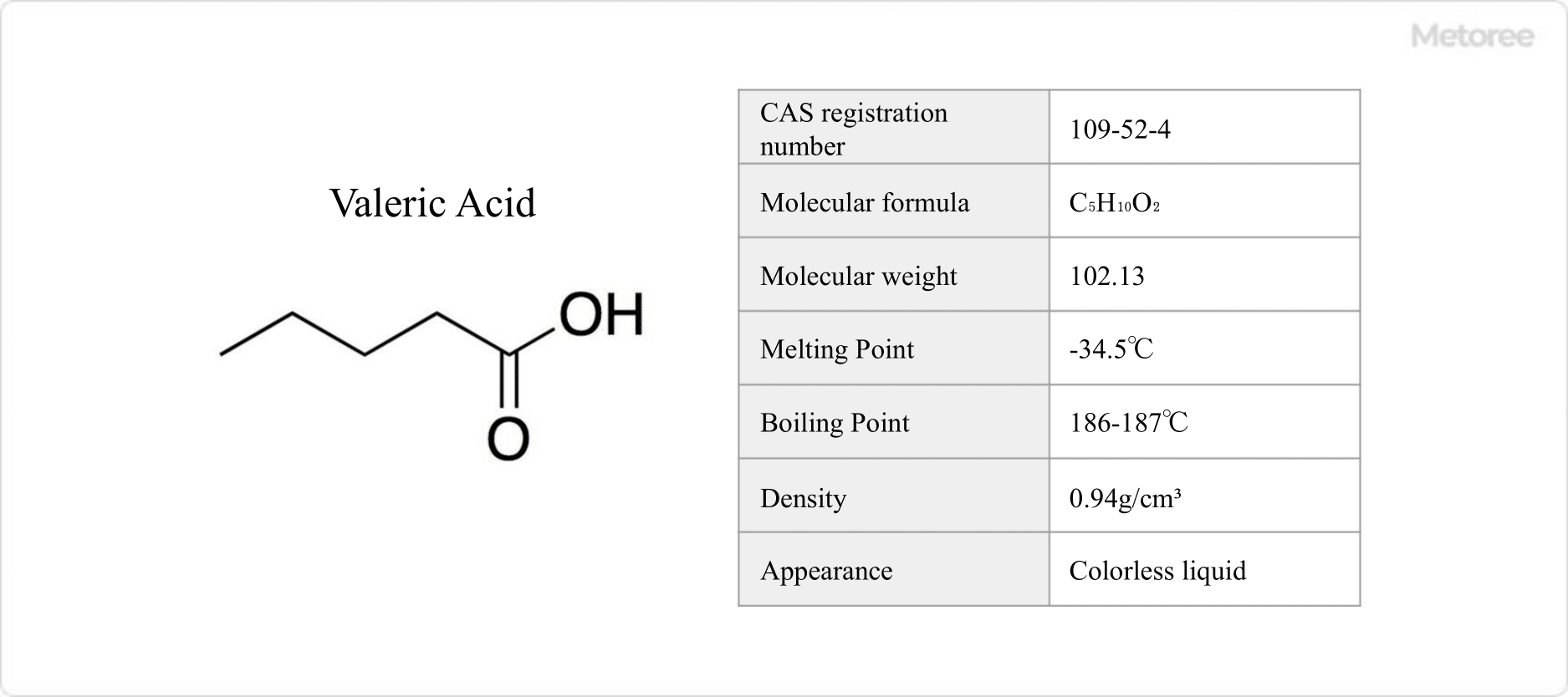
Figure 1. Basic information on valeric acid
Valeric acid is a 5-carbon saturated chain carboxylic acid.
Also known as pentanoic acid, it has an unpleasant odor and was first discovered in the European herb Valerian. The dried root of Valerian has been used medicinally for centuries.
Foot odor is caused by isovaleric acid, which is an isomer of valeric acid.
Uses of Valeric Acid
Valeric acid is widely used in food flavoring, particularly in the form of esters like butyl valeric acid and pentyl valeric acid.
Although valeric acid itself has an unpleasant aroma, in small quantities it can contribute a fruity flavor and is used in fruit essences and essential oils.
It is used as a flavoring agent in various flavors, such as apple, peach, and apricot, at concentrations ranging from 4.2 to 15 ppm, and in chewing gum at about 260 ppm.
Properties of Valeric Acid
Valeric acid is well soluble in ethanol and ether, but only slightly soluble in water. It represents the lowest molecular weight carboxylic acid that is soluble in non-polar solvents. It is weakly acidic, with a pKa of 4.82, and dissolves in alkali carbonate and alkali hydroxide solutions to form salts. It is corrosive to human tissue.
Valeric acid has a melting point of -30 °F (-34.5 °C) and a boiling point of 366.8-368.6 °F (186-187 °C). Its odor is often likened to steaming socks.
Structure of Valeric Acid
The chemical formula of valeric acid is C5H10O2, with a molecular weight of 102.13 g/mol. Its structure is CH3(CH2)3COOH, and it has a density of 0.94 g/cm3. At physiological pH, it forms C4H9COO-, the conjugate base of valeric acid.
As a carboxylic acid, valeric acid reacts with alcohols to form esters and can also be used to synthesize amides, anhydrides, and acid chlorides. Pentanoyl chloride, a commonly used acid chloride, is often used as an intermediate in synthesis.
Other Information on Valeric Acid
1. Valeric Acid Synthesis
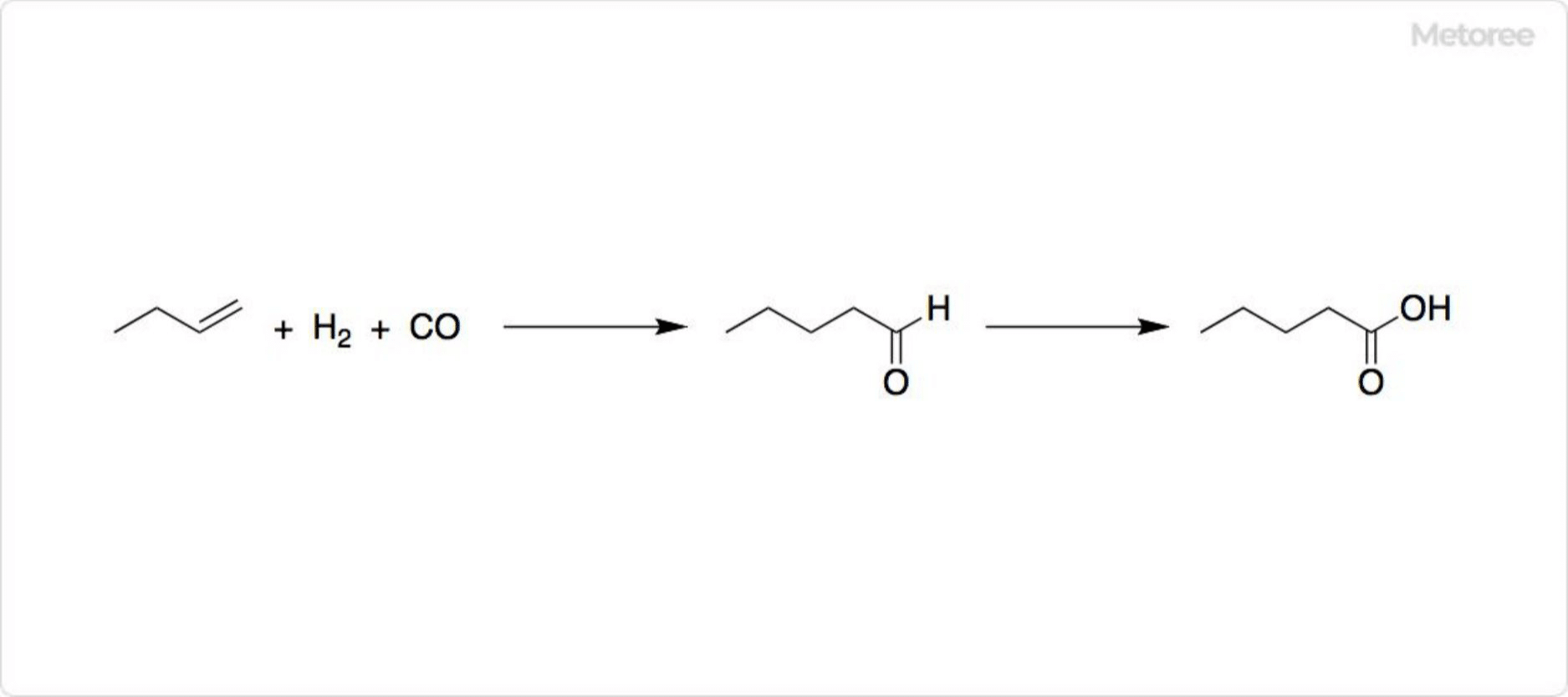
Figure 2. Synthesis of valeric acid
Valeric acid can be produced by the hydrolysis of valeronitrile and is also synthesized via the oxidation of n-amyl alcohol (1-pentanol).
In industrial processes, butyraldehyde is formed from 1-butene and syngas through hydroformylation, followed by oxidation to produce valeric acid.
Biomass-derived sugars can be converted to valeric acid via levulinic acid, a method gaining attention for biofuel production.
2. Structural Isomers of Valeric Acid
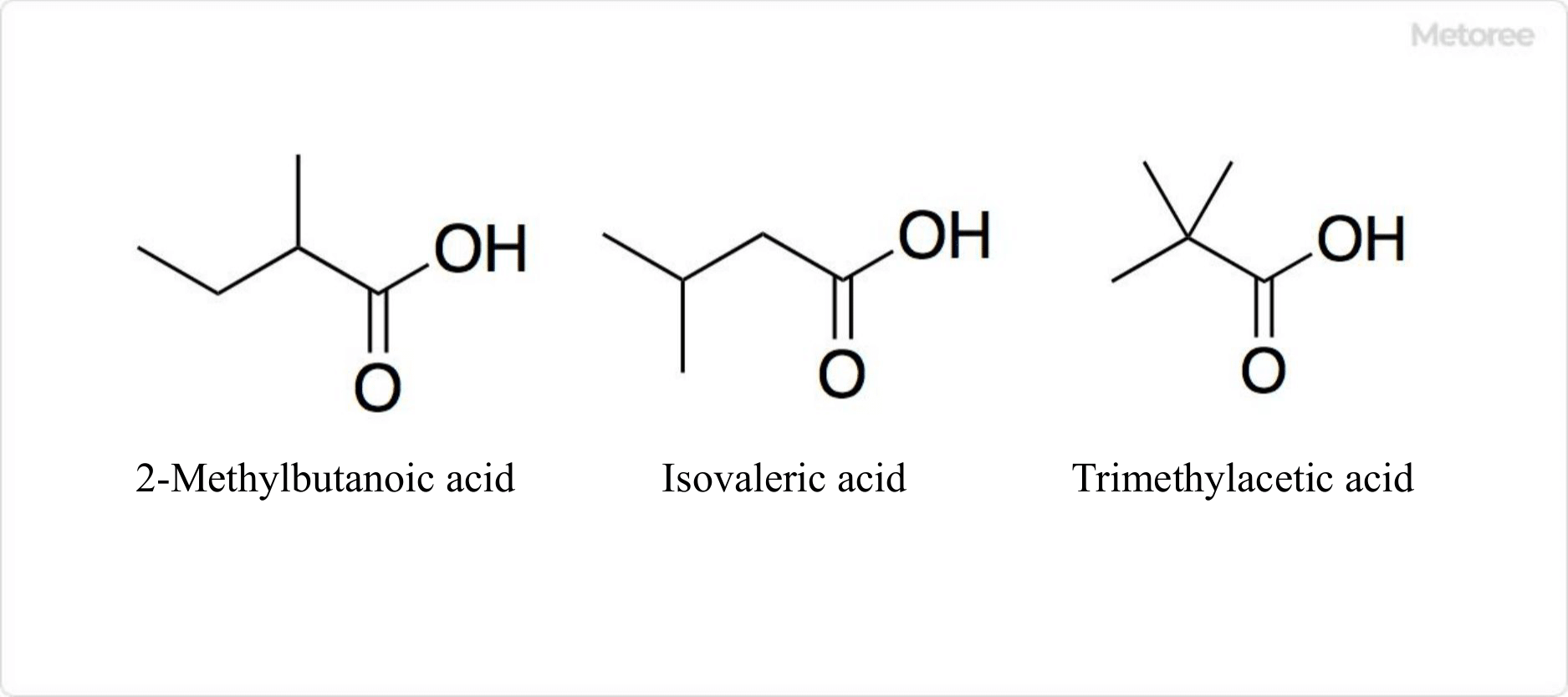
Figure 3. Structural isomers of valeric acid
The structural isomers of valeric acid include trimethylacetic acid, isovaleric acid, and 2-methylbutanoic acid. Trimethylacetic acid is also known as pivalic acid and neopentanoic acid, isovaleric acid as 3-methylbutanoic acid, and 2-methylbutanoic acid as hydroangelic acid.
3. Characteristics of the Structural Isomers of Valeric Acid
The specific formula of trimethylacetic acid is (CH3)3CCOOH, with a density of 0.905 g/cm3. Its melting point is 95.9 °F (35.5 °C) and the boiling point is 326.8 °F (163.8 °C).
Isovaleric acid’s formula is (CH3)2CHCH2COOH. It is the most common structural isomer of valeric acid found in nature and occurs in the roots of the Ominaceae species, valericaceae. It has a density of 0.925 g/cm3, a melting point of -20 °F (-29 °C), and a boiling point of 347-350.6 °F (175-177 °C).
2-methylbutanoic acid, with the formula C2H5(CH3)CHCOOH, has two optical isomers: (R)-2-methylbutanoic acid and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid is found in cocoa beans, while (S)-2-methylbutanoic acid is present in many fruits, including apples and apricots. Its density is 0.94 g/cm3, with a melting point of -130 °F (-90 °C), and a boiling point of 348.8 °F (176 °C).