What Is Tyramine?
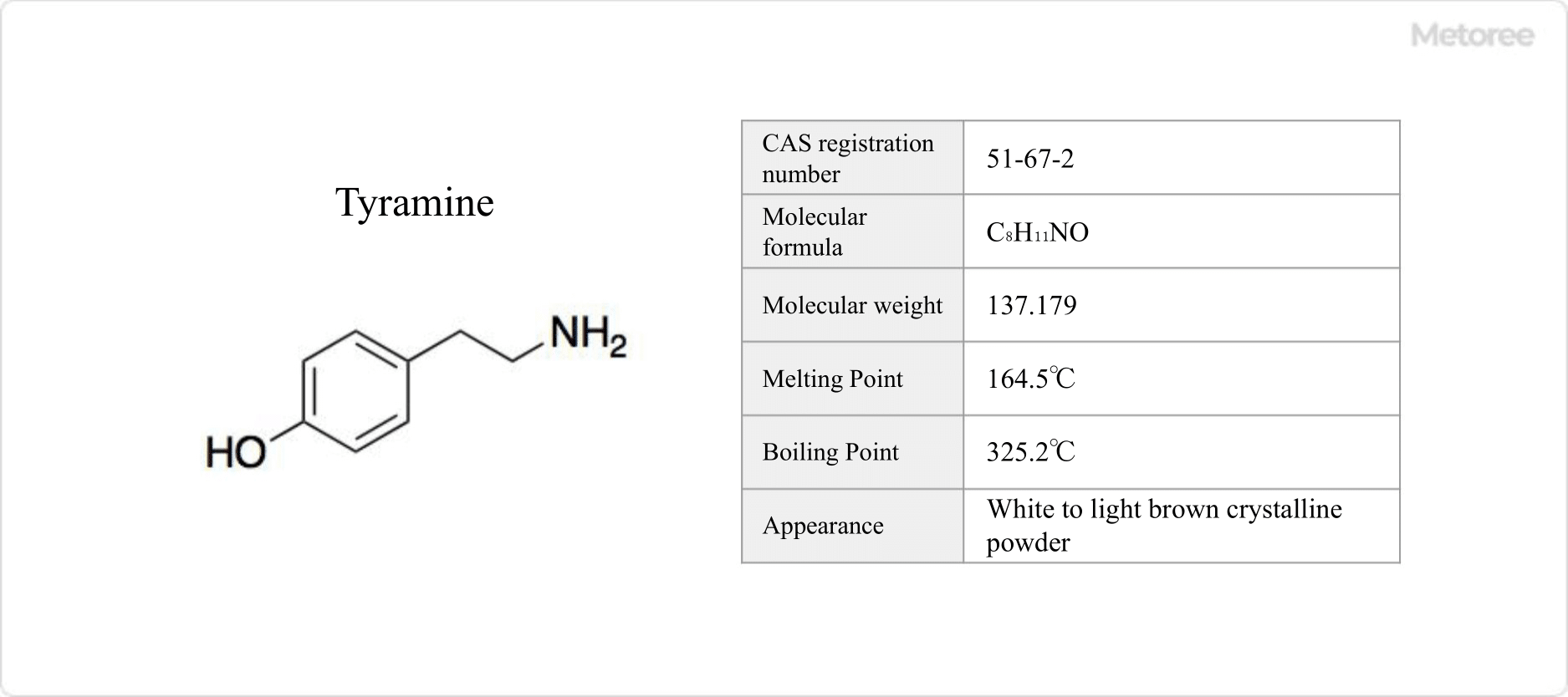
Figure 1. Basic Information on Tyramine
Tyramine is a derivative of phenethylamine (phenylethylamine).
It is also known as p-tyramine, utheramine, tocosine, tyrosamine, and cystogen. Foods high in tyramine, such as aged cheeses, red wine, cocoa products, fermented foods, pickled foods, and smoked foods, can trigger hypertensive crises.
Tyramine is found extensively in animal and plant organisms and is produced by enzymatic action. It is metabolized and deactivated by monoamine oxidase enzymes.
Uses of Tyramine
As a monoamine, tyramine has several physiological effects, including raising blood pressure due to its vasoconstrictive properties.
It is used in immunofluorescence analysis as a substrate for peroxidase, marked with fluorescent dyes.
Properties of Tyramine
Tyramine appears as a white to light brown crystalline powder, soluble in ethanol and water, and virtually insoluble in acetone. Its melting point is 164.5°C, and its boiling point is 325.2°C.
The simultaneous consumption of tyramine-rich foods and the antituberculosis drug isoniazid may lead to side effects such as sweating, headaches, abdominal pain, and increased blood pressure. Phenylpropanolamine hydrochloride, a medication for rhinitis, may cause similar effects.
Structure of Tyramine
Tyramine, a phenethylamine derivative, has a hydroxy group at the phenyl group’s 4-position, also known as 4-hydroxyphenethylamine or p-hydroxyphenethylamine.
Its chemical formula is C8H11NO, with a molar mass of 137.179 g/mol and a density of 1.103 g/cm3.
Other Information on Tyramine
1. Biosynthesis of Tyramine
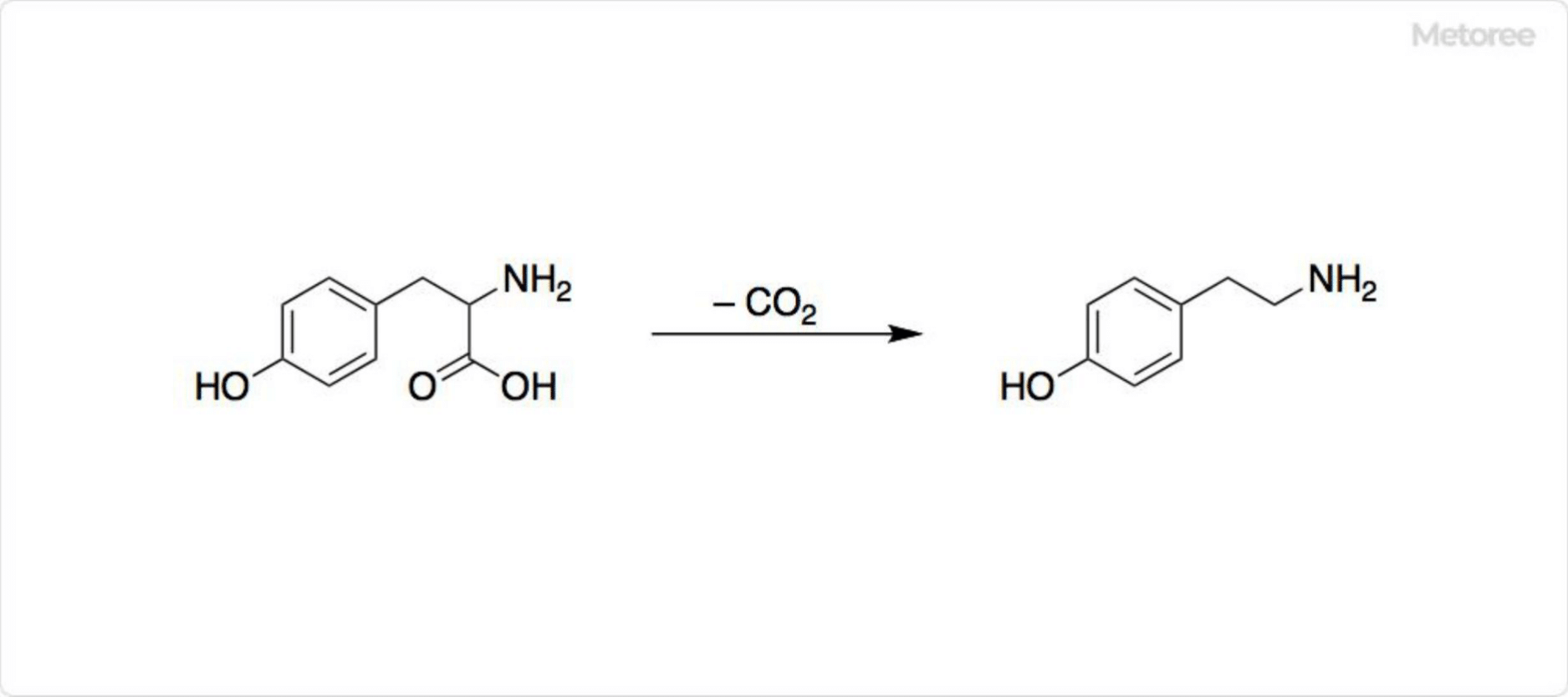
Figure 2. Biosynthesis of Tyramine
Tyramine, widely present in plants and animals, is biosynthesized in humans from L-phenylalanine (Phe). Tyrosine (Tyr) can be synthesized from phenylalanine in vivo by phenylalanine-4-monooxygenase and its coenzyme tetrahydrobiopterin. Aromatic L-amino acid decarboxylase then decarboxylates tyrosine to produce tyramine. Tyramine is also generated as a putrefactive amine when proteins in food are degraded by microorganisms.
2. Action of Tyramine
Tyramine stimulates the release of noradrenaline, a vasoconstrictor, leading to increased blood pressure. This can contribute to migraine attacks and an increased heart rate. However, repeated short-term administration of tyramine depletes noradrenaline, reducing its effectiveness, as noradrenaline biosynthesis cannot keep pace.
High-tyramine foods, including cocoa and chocolate, can sometimes cause headaches due to blood vessel dilation after the vasoconstrictive effects of tyramine subside.
3. Compounds Related to Tyramine
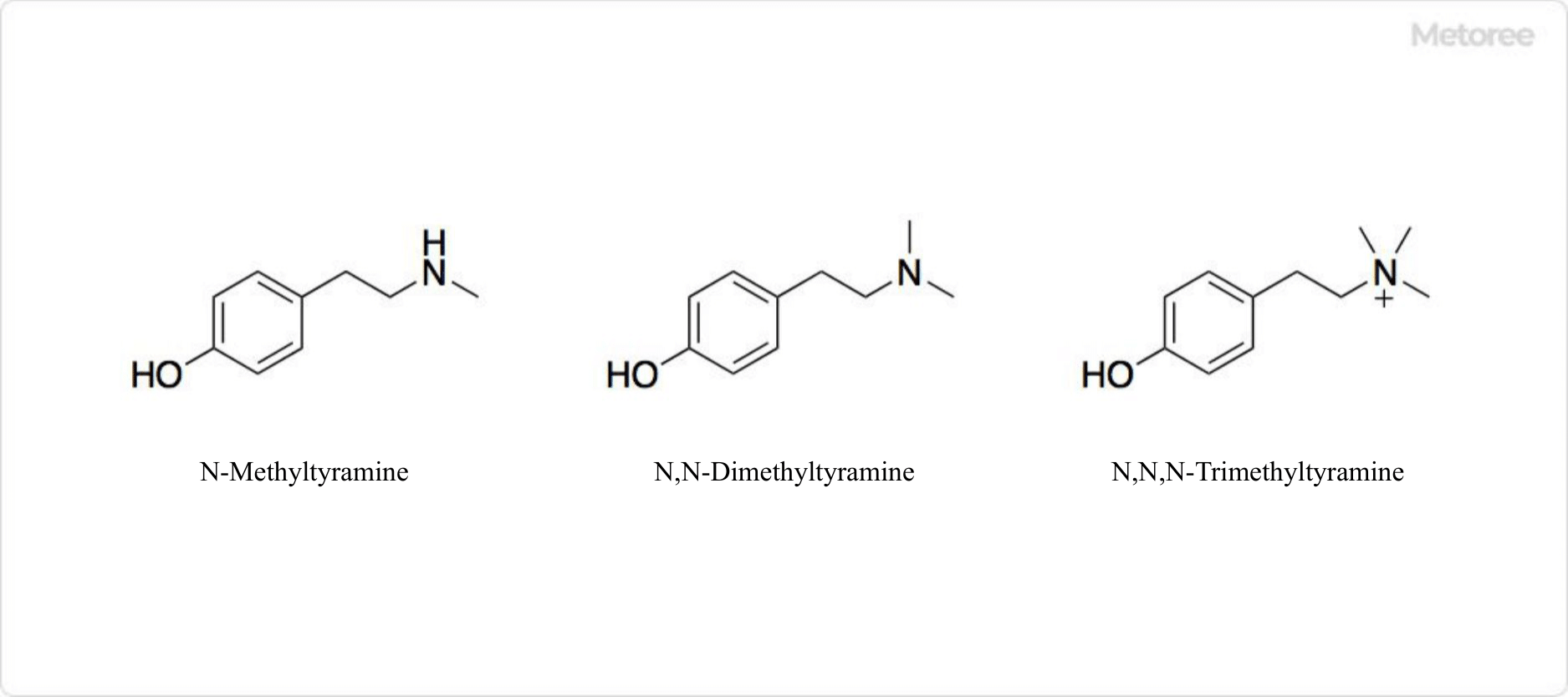
Figure 3. Related Compounds of Tyramine
Tyramine can be methylated to produce alkaloids such as N-methyltyramine, N,N-dimethyltyramine, and N,N,N-trimethyltyramine. Alkaloids, generally, are organic compounds containing nitrogen atoms; N,N-dimethyltyramine is also known as hordenine, and N,N,N-trimethyltyramine is known as candicine.
Tyramine is structurally similar to monoamine neurotransmitters, such as adrenaline, noradrenaline, histamine, dopamine, serotonin, and acetylcholine, and shares similar physiological effects, including uterine contraction, blood pressure elevation, and peripheral nerve contraction.