What Is Lithium Aluminum Hydride?
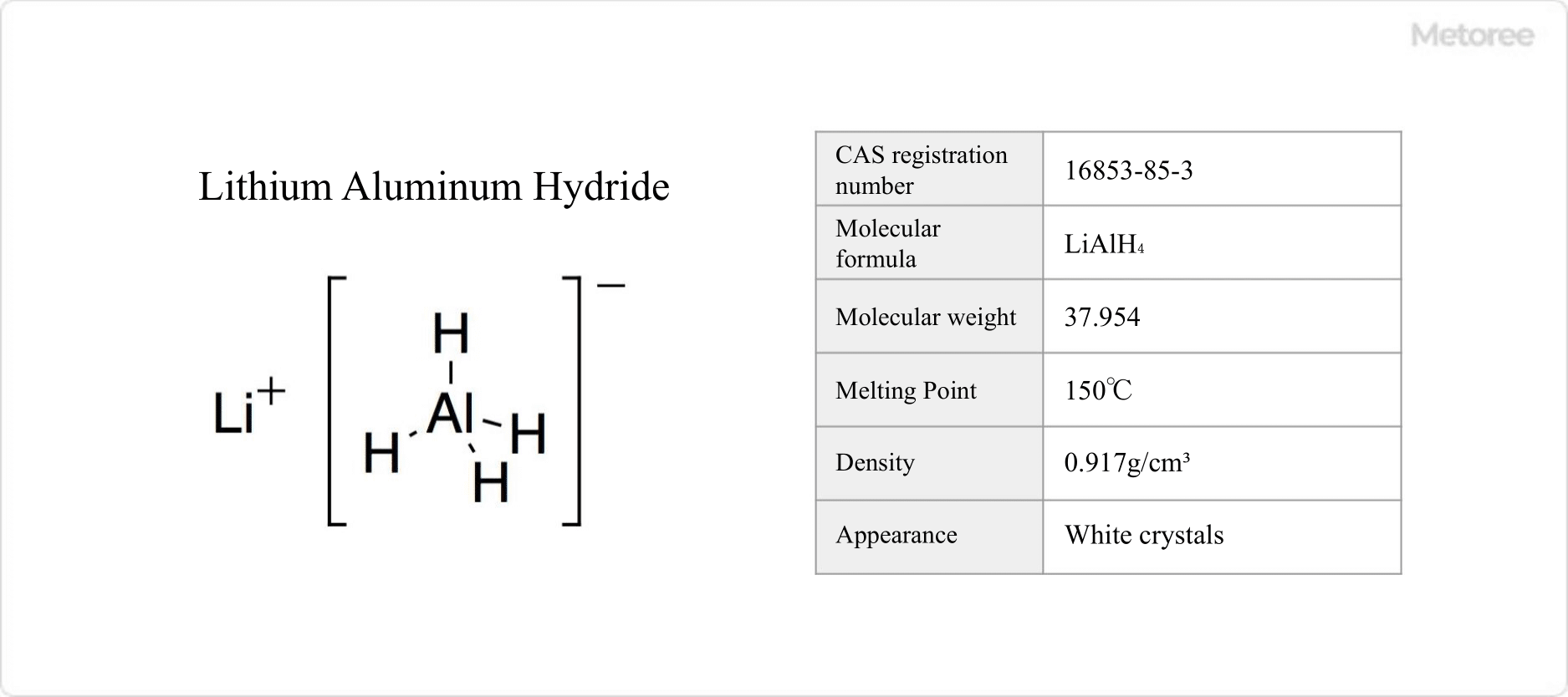
Figure 1. Basic Information on Lithium Aluminum Hydride
Lithium aluminum hydride, an inorganic compound with the formula LiAlH4, is a potent reducing agent. It is recognized for its diverse applications in chemical synthesis, despite being classified as a hazardous material due to its reactivity with water and potential for flammability and toxicity.
Uses of Lithium Aluminum Hydride
Primarily, lithium aluminum hydride serves as a reducing agent in chemical synthesis, favored in laboratories for its high reactivity. In industrial applications, its use is more cautious due to safety concerns, favoring milder reductants instead.
Its main application includes the reduction of esters and carboxylic acids to primary alcohols, employing dehydrated ether as the solvent.
Properties of Lithium Aluminum Hydride
This compound has a molar mass of 37.954 g/mol and a density of 0.917 g/cm3. Its superior reducing power, compared to sodium borohydride, stems from the weaker Al-H bonds, facilitating vigorous reactions with water to produce hydrogen gas.
Structure of Lithium Aluminum Hydride
As an ionic crystal, lithium aluminum hydride comprises aluminum hydride ions (AlH−4) and lithium ions (Li+). It crystallizes in the monoclinic system, with the AlH4− ions adopting a tetrahedral geometry and an Al-H bond distance of approximately 1.55 Å.
Other Information on Lithium Aluminum Hydride
1. Synthesis
The synthesis of lithium aluminum hydride involves reacting aluminum chloride (AlCl3) with lithium hydride (LiH), yielding approximately 97%. The product can be purified from lithium chloride (LiCl) by dissolving the reaction mixture in ether and filtering off the insoluble solids.
2. Decomposition Reaction
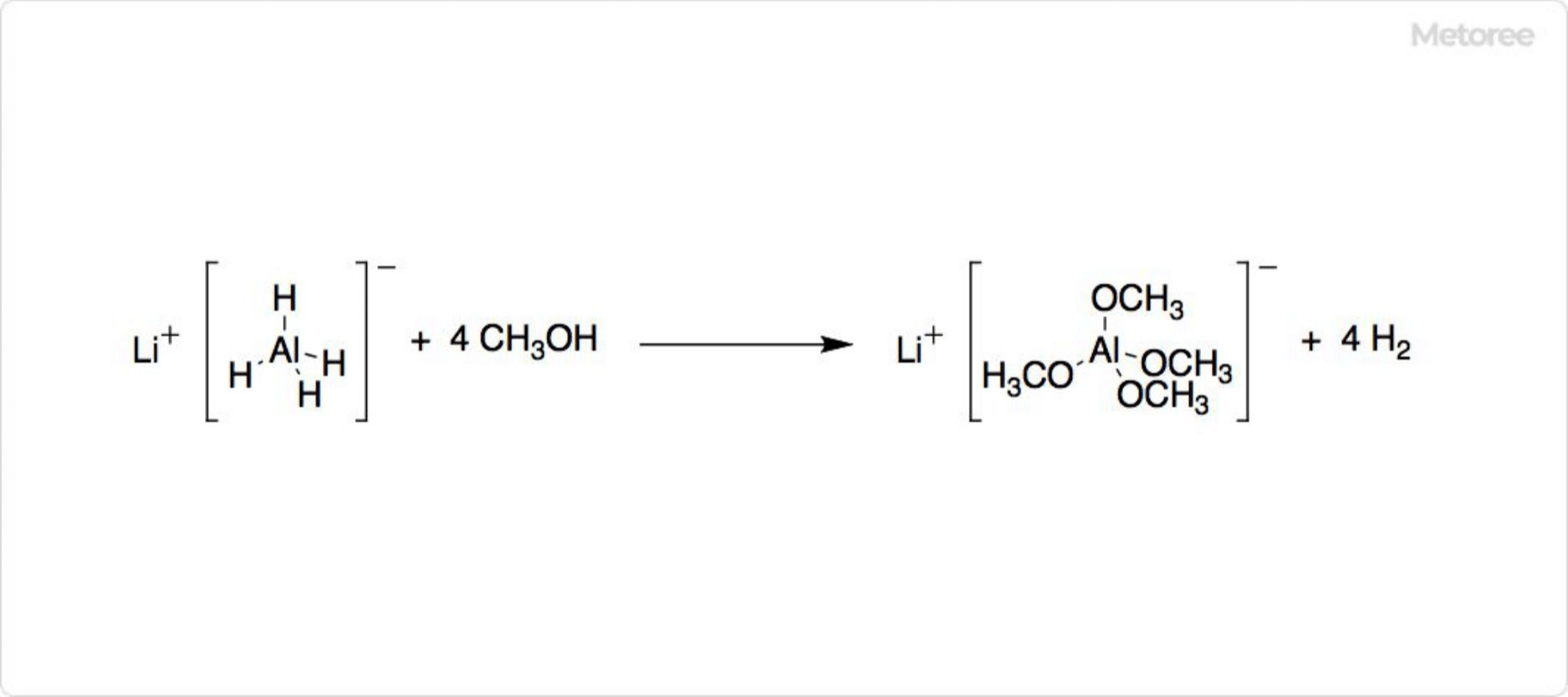
Figure 2. Decomposition Reaction of Lithium Aluminum Hydride
Lithium aluminum hydride’s instability in the presence of protic solvents, such as alcohols, leads to its violent decomposition. It is metastable at room temperature, gradually decomposing into Li3AlH6 and LiH, a process that is expedited by the presence of certain metals.
3. Inorganic Reactions
In reactions with sodium hydride in tetrahydrofuran (THF), lithium aluminum hydride yields sodium aluminum hydride (NaAlH4). This process is reversible and can also produce potassium aluminum hydride (KAlH4) with high efficiency.
4. Organic Reactions
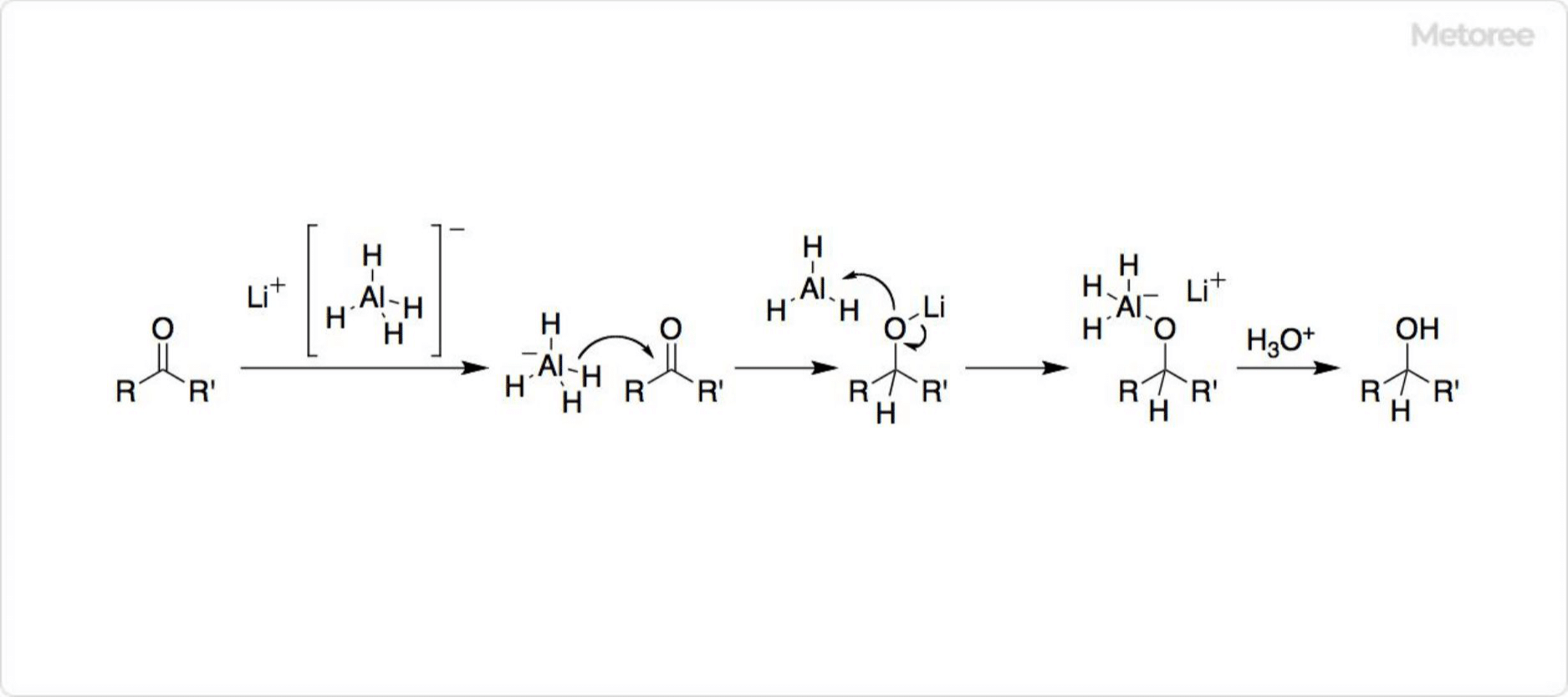
Figure 3. Reaction Mechanism of Lithium Aluminum Hydride
In organic synthesis, lithium aluminum hydride is esteemed for its ability to reduce esters and carboxylic acids to primary alcohols through the action of the hydride ion, H−, which attacks the electrophilic centers of molecules.