What Is Copper Sulfate?
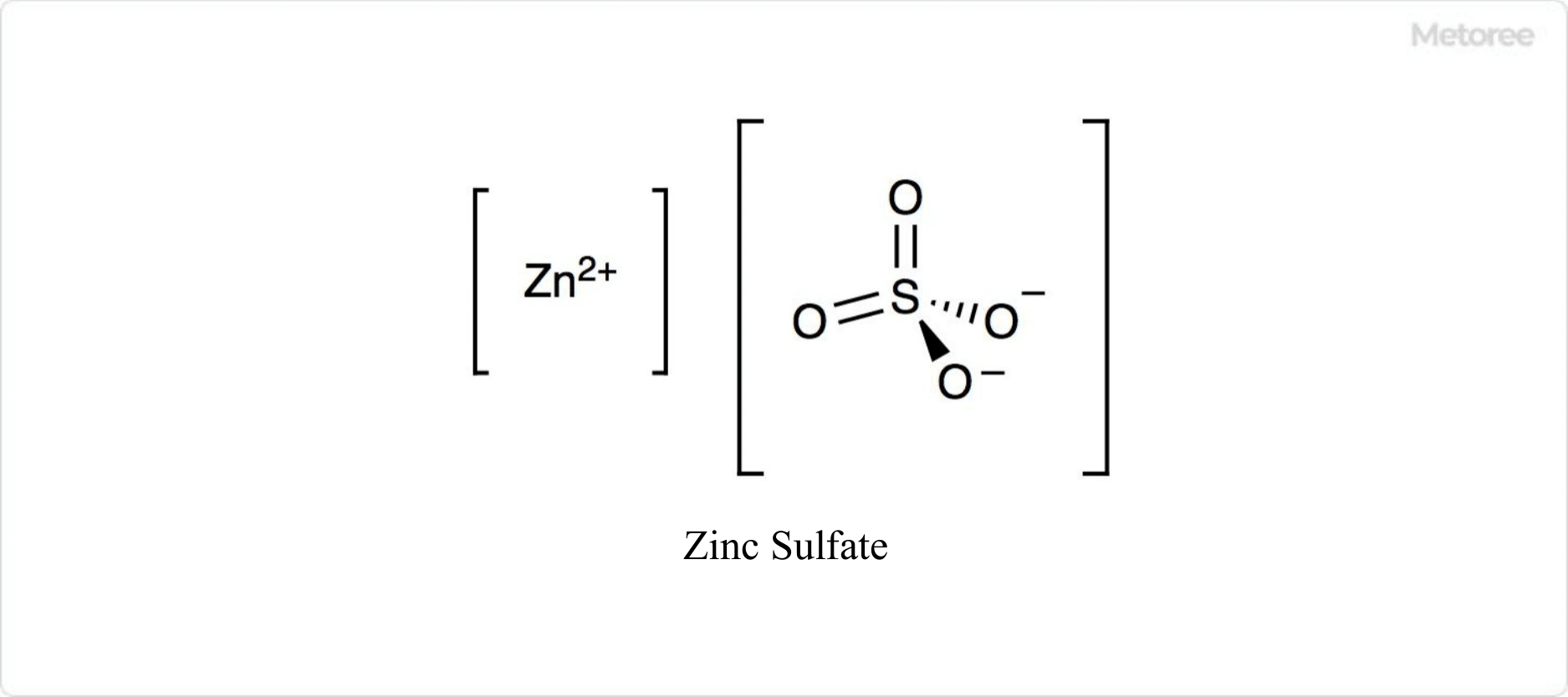
Copper sulfate, consisting of monovalent copper (I) and divalent copper (II), is a significant chemical compound used for various applications. The chemical formula for copper (I) sulfate is Cu2SO4, while for copper (II) sulfate, it is CuSO4. Typically, copper sulfate refers to the copper (II) form. Copper (I) sulfate appears as a colorless or gray powder, reacting readily with water and decomposing.
Copper (II) sulfate exists in both anhydrous and pentahydrate forms. The anhydrous form is white crystal that easily absorbs water to form the pentahydrate. The pentahydrate, naturally occurring as chalcanthite, is blue and is industrially produced by treating brass ore with air and water.
Uses of Copper Sulfate
Applications of copper sulfate include its use in the Bordeaux mixture, an agricultural fungicide made from copper sulfate and slaked lime. This solution is effective against bacterial and fungal diseases on fruit trees and vegetables. Additionally, copper sulfate is vital in infant formula to meet copper nutritional requirements, within standards set by regulatory authorities. It is also used in copper plating, as a catalyst, and in pigments.
Properties of Copper Sulfate
The anhydrous form is a white, water-soluble, hygroscopic powder that decomposes into copper oxide and sulfur trioxide at 650°C. The pentahydrate form, a blue crystal, dissolves in water, with its solubility varying with temperature.
The repeated information on the solubility of the pentahydrate form has been removed for conciseness.
Structure of Copper Sulfate
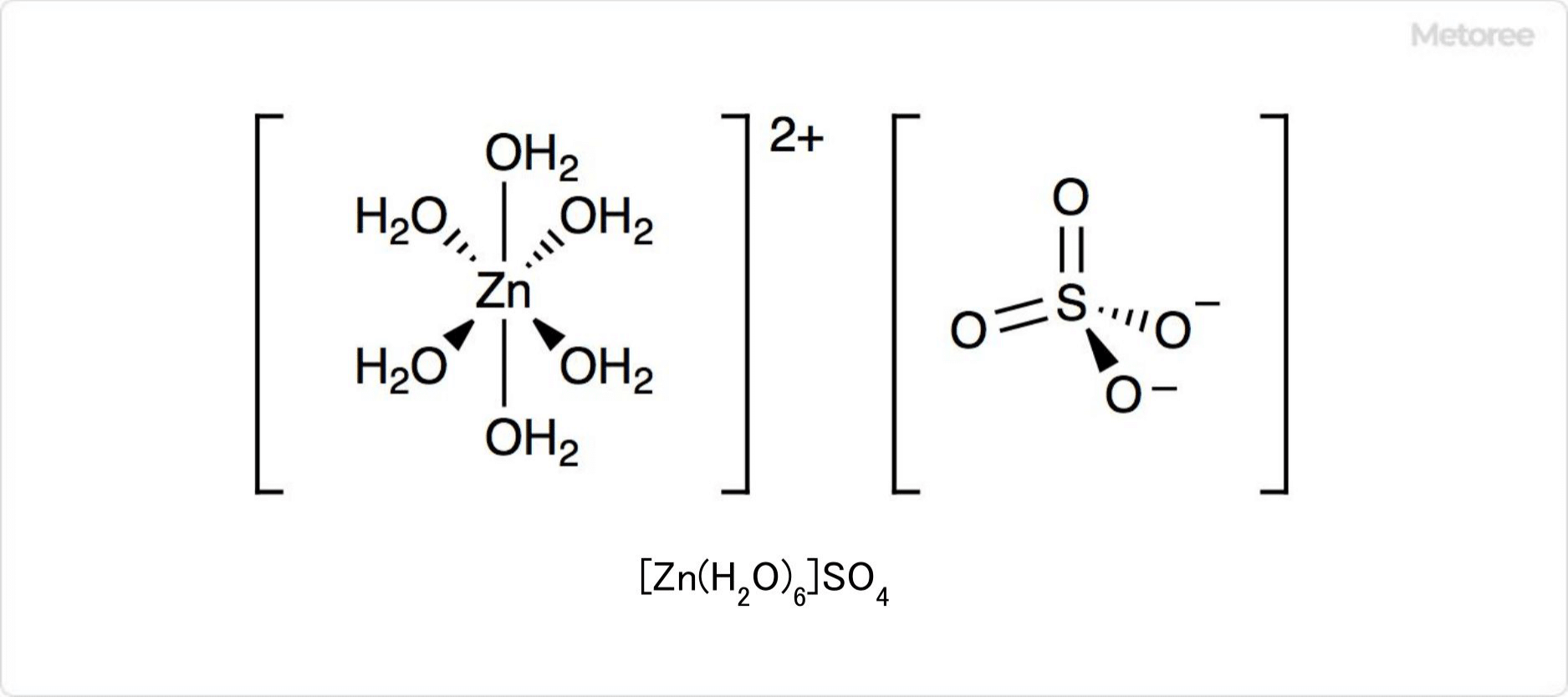
The structure of copper sulfate pentahydrate includes five molecules of water, with four directly coordinated to the copper ion. The sulfate ion is weakly coordinated, and the crystal structure allows for hydrogen bonding among water molecules and sulfate ions. Heating the pentahydrate leads to sequential water loss, altering the crystal structure and coordination.
Other Information on Copper Sulfate
1. Copper Sulfate Production Method
Copper sulfate pentahydrate is produced industrially by dissolving copper compounds in sulfuric acid and evaporating the solution. Anhydrous copper sulfate is made by dehydrating the pentahydrate, with careful temperature control to prevent decomposition into other compounds.
- Dehydration: CuSO4・5H2O → CuSO4・3H2O → CuSO4・H2O → CuSO4
- Decomposition: CuSO4 → CuO + SO2 + O2
2. Safety Information on Copper Sulfate
Copper sulfate poses significant health risks, including acute toxicity through inhalation or ingestion. Symptoms of copper sulfate poisoning can include gastrointestinal distress, hemolytic anemia, and potential damage to the liver and kidneys.