What Is a Potentiostat?
A potentiostat is a device that controls electric potential and current and measures electric potential.
In a three-electrode system consisting of a working electrode, a counter electrode, and a reference electrode in an electrolyte, it controls the voltage between the working electrode and the reference electrode and measures the current flowing between the working electrode and the counter electrode. It is used in electrochemical measurements and is often used in conjunction with galvanostats and function generators.
While galvanostats accurately control the current flowing through the electrode and arbitrarily regulate the electrochemical rate, potentiostats control the potential of the electrode and measure the current flowing through the electrode at that time. Potentiostats are used to measure the current flowing through an electrode.
Uses of Potentiostats
Potentiostats are mainly used for electrochemical measurements. In practice, potentiostats are rarely used alone but are most often used in combination with galvanostats and function generators.
Potentiostats alone can only perform constant voltage control, but when combined with these devices, they can perform voltage sweep operations and pulse outputs, and their response can be measured to gain a deeper understanding of the electrochemical characteristics of an object.
Principle of Potentiostats
The key to the potentiostat’s principle is its negative feedback control using an operational amplifier.
The operational amplifier is represented by the circuit symbol shown in Figure 1 and has the following two characteristics:
- Very large internal impedance
- The voltage at the positive and negative input terminals can be regarded as the same (VIN1=VIN2)
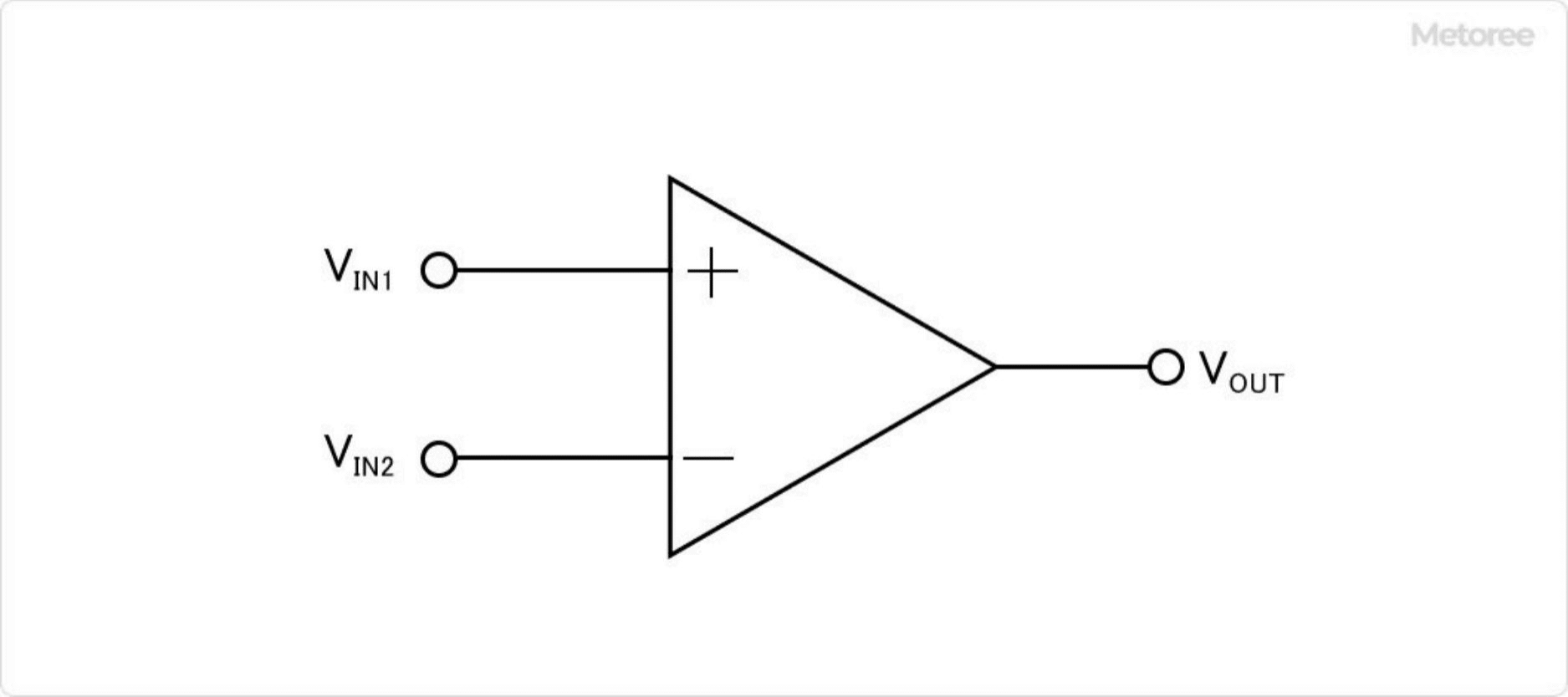
Figure 1. Circuit symbols for operational amplifiers
The following major functions of the potentiostats are achieved by the circuit using an operational amplifier:
- Prevents current flow to the reference pole
- Controls the potential of the working electrode with respect to the reference electrode
- Measures the current flowing between the working electrode and the counter electrode
The high internal impedance prevents current from flowing to the reference pole, and since the voltage at the positive and negative ends is the same, the set voltage can be used as the voltage at the reference pole as it is.
Since the operational amplifier outputs a voltage corresponding to the flowing current, the current can be measured.
Other Information on Potentiostats
1. What Is Electrochemical Measurement?
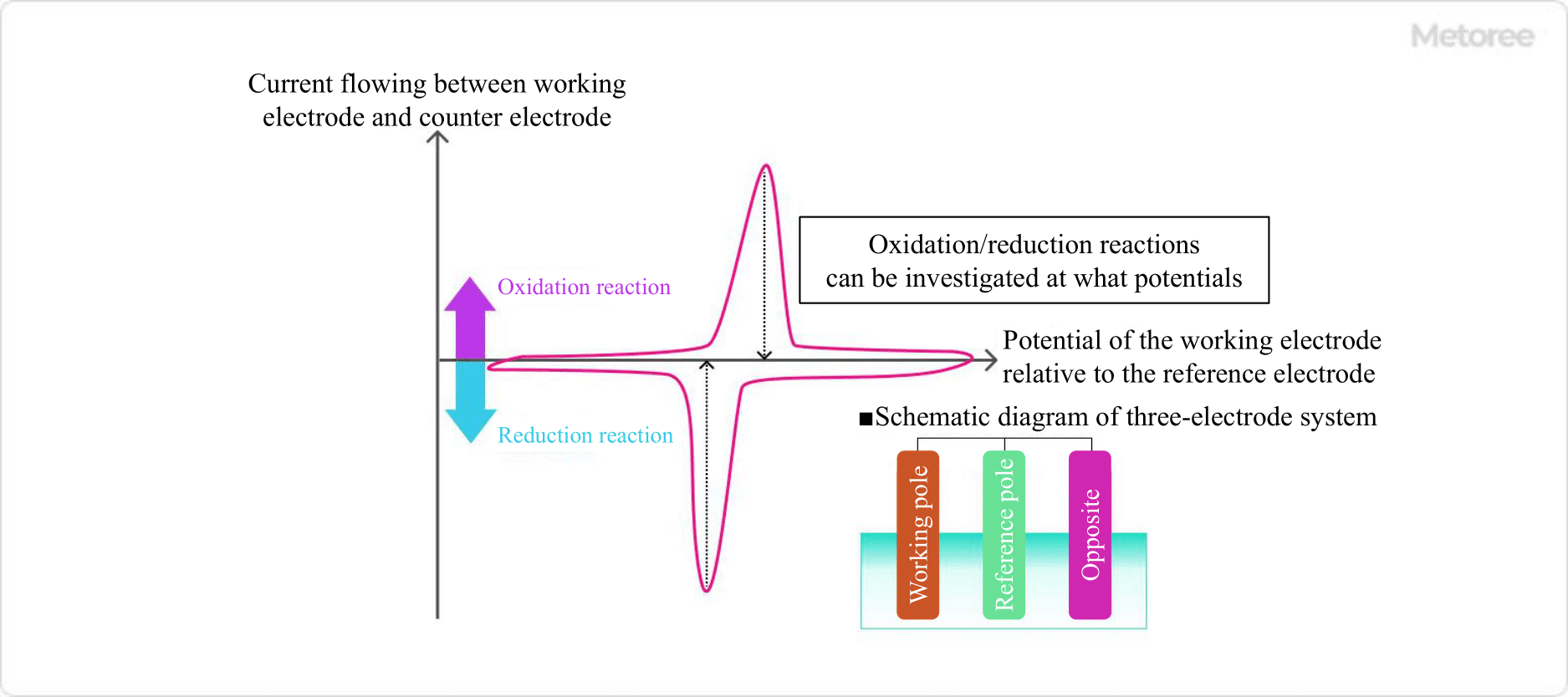
Figure 2. Example of voltammogram
Electrochemical measurement is a measurement technique in which an electrical signal is applied to a specific sample from a power source or another circuit to induce a chemical reaction, and the response signal is used to evaluate the chemical reaction occurring inside.
A common example is the electrolysis of water. By inserting electrodes connected to potentiostats into water and applying electrical energy from an external circuit, the electrolysis reaction of water can be promoted or reversed on the working electrode. This technique can be utilized in the development of catalysts that promote the electrolysis of water.
In the voltammogram measurement, a computer is used to convert the controlled electrode potential from a digital signal to an analog signal, and the electrolytic current measured by the potentiostats is digitized and read by the computer. In this way, it is possible to determine how much current flowed in relation to the voltage change.
From the voltammogram, it is possible to determine what kind of oxidation/reduction reaction is taking place at the working electrode at each potential.
2. Reasons for Using a Three-Electrode System
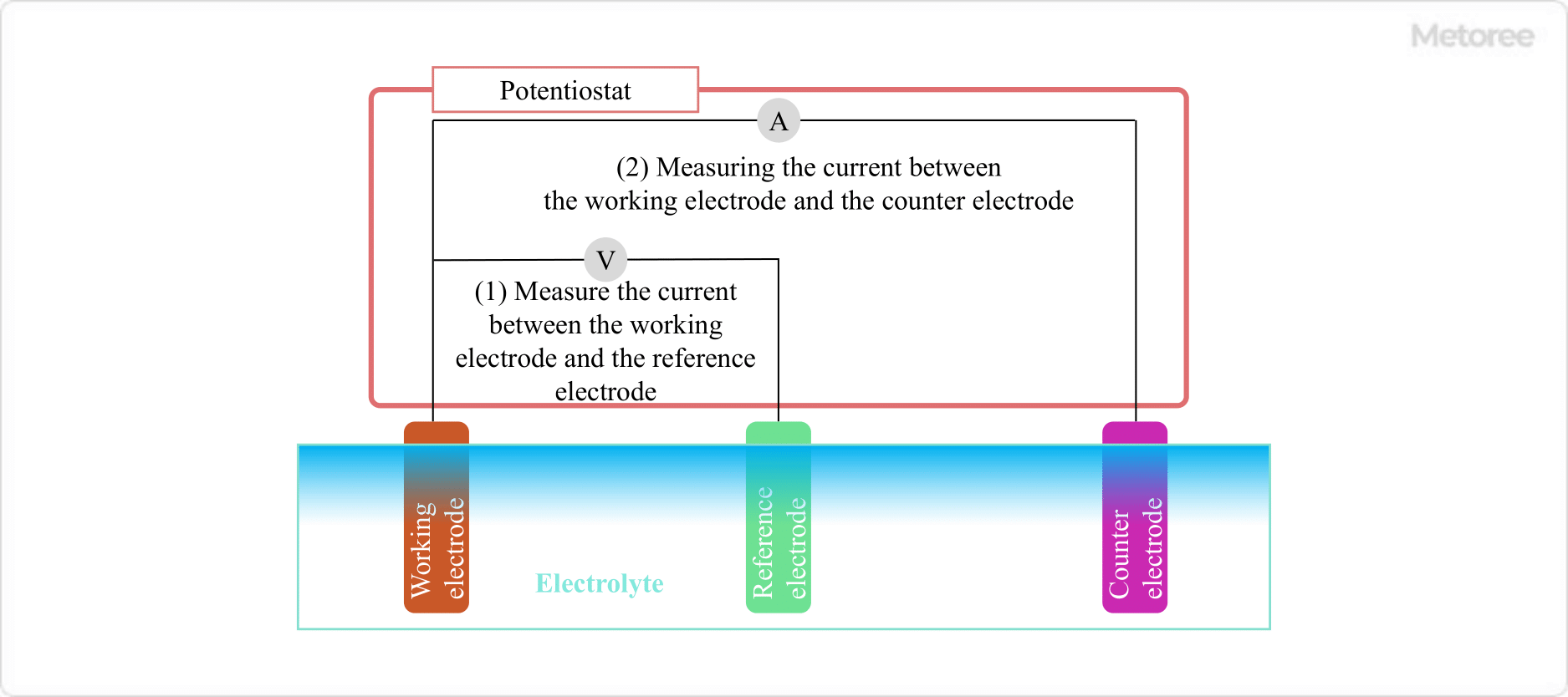
Figure 3. Schematic diagram of potentiostat and three-electrode system
In electrochemical measurements, it is necessary to accurately determine the potential of reactions occurring at the electrodes. 2-electrode electrochemical measurements cannot accurately measure the potential due to the phenomenon of polarization, which occurs when current flows through both the working and counter electrodes. Polarization refers to a shift in electrode potential due to current flow in the circuit.
Therefore, a reference electrode is added as a third electrode to form a three-electrode system consisting of an action electrode, a counter electrode, and a reference electrode. No current flows through the reference electrode, and by measuring the potential difference between the working electrode and the reference electrode, the potential of the working electrode can be accurately measured.