What Is Phosphorous Acid?
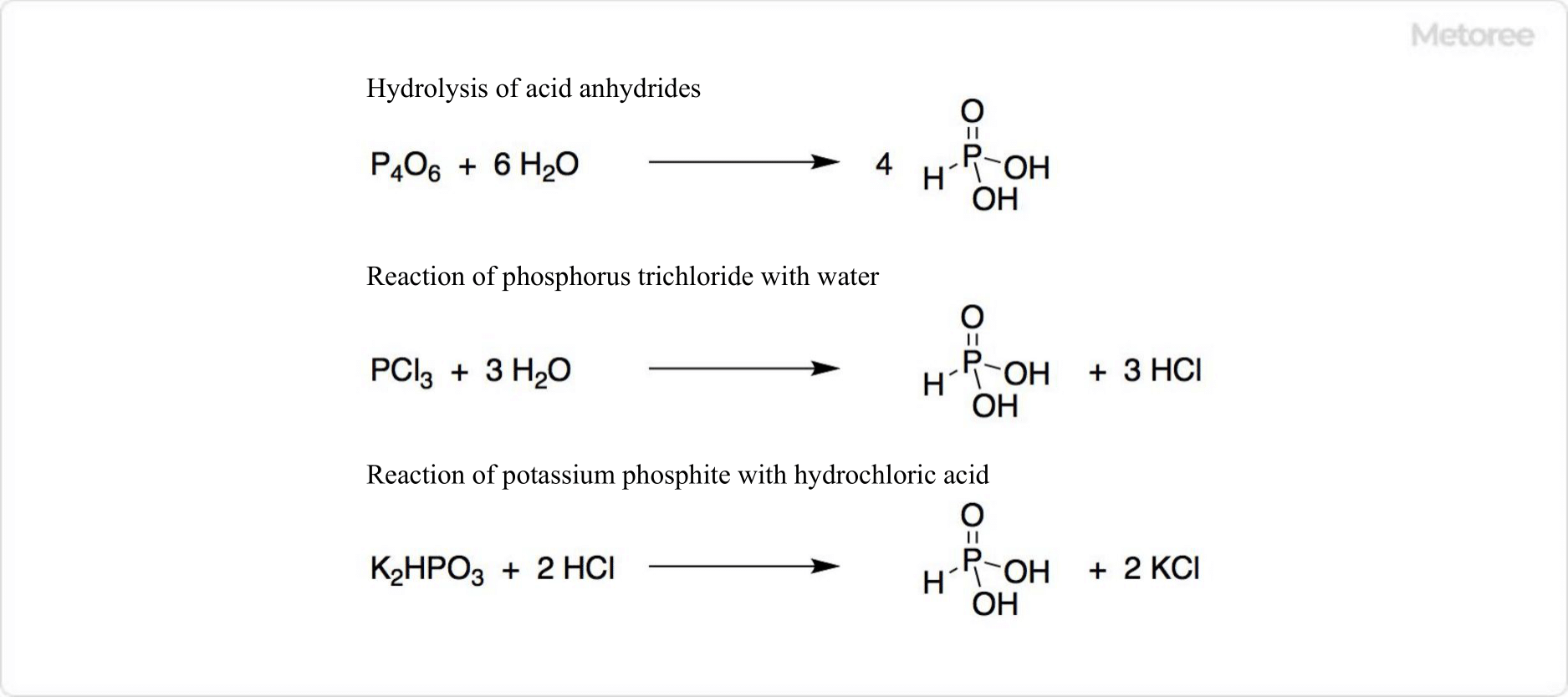
Figure 1. Synthesis of Phosphorous Acid
Phosphorous acid is an inorganic phosphorus oxoacid, chemically represented by H3PO3. It belongs to the family of phosphorus oxoacids, including phosphoric acid and hypophosphorous acid. Phosphorous acid can be synthesized through the hydrolysis of acid anhydrides, typically by reacting phosphorus trichloride with water or steam. Notably, it has gained attention for its application in fertilizers. Pure phosphorous acid can be extracted from potassium phosphorous acid by treating it with hydrochloric acid, followed by concentration with alcohol or precipitation.
Uses of Phosphorous Acid
Phosphorous acid serves multiple roles, including as a catalyst and reducing agent in organic compound synthesis and as a stabilizer in vinyl chloride production. It is also a precursor for phosphite fertilizers. Despite phosphoric acid being a common component in phosphate fertilizers, phosphorous acid has emerged as an alternative due to its lower molecular weight, better solubility, and superior crop absorption and soil adsorption characteristics. This acid is found in both granular and liquid fertilizers, often combined with potassium, enhancing crop yield and quality when used correctly.
Properties of Phosphorous Acid
At room temperature and pressure, phosphorous acid appears as stable white crystals but is deliquescent. In aqueous solution, it primarily exists as HP(O)(OH)2, demonstrating divalent acidity with a pH around 1. Its pKa1 is 1.5 and pKa2 is 6.79. Phosphorous acid is noted for its strong reducing properties, capable of liberating metals from solutions like silver nitrate, gold (III) chloride, and copper sulfate, with a standard redox potential of E° = -0.276 V in acidic solutions.
Structure of Phosphorous Acid
Phosphorous acid exhibits tautomerism, with hydrogen atoms shifting between phosphorus and oxygen. The predominant form is HP(O)(OH)2, also known as phosphonic acid, over its trihydroxy counterpart, P(OH)3. In solid form, phosphorous acid adopts a tetrahedral structure, with specific bond lengths for P-H, P-O, and P-O(H) bonds.
Other Information on Phosphorous Acid
1. Isomers of Phosphorous Acid
In solution, phosphorous acid exists in equilibrium with its tautomer, phosphonic acid, though phosphorous acid is the more prevalent form. In the realm of organic chemistry, phosphonic acids refer to compounds with the formula R-P(=O)(OH)2, including substances like foscarnet, an antiviral drug. The diesters of P-alkylphosphonic acid are key substrates in the Horner-Wadsworth-Emmons reaction, pivotal for synthesizing alkenes.
2. Phosphorous Acid Related Compounds
Phosphorous acid is part of the broader phosphorus oxoacid family. Phosphorus oxoacids with an oxidation state of +5 include phosphoric acid, diphosphoric acid, triphosphoric acid, and meta-triphosphoric acid, among others. Phosphinic acid, with a phosphorus oxidation state of +1, is another related compound, expressed as H3PO2.