What Is an Infrared Spectrophotometer?

Figure 1. Infrared spectrophotometer and IR spectrum image
An infrared spectrophotometer is an analytical instrument that irradiates a sample with infrared light and detects the transmitted and reflected infrared light.
It is used to obtain information about the molecular structure of a sample. The primary components of the device include a light source, spectrometer, sample, and detector. When a molecule is irradiated with infrared light, absorption occurs due to the vibration and rotation of the molecules in the sample. Since this absorption spectrum differs depending on the molecular structure, it is possible to obtain information about the molecular structure.
It is especially used to identify functional groups in molecular structures and for qualitative and quantitative analysis of samples. This method is non-destructive and easy to use and can be applied to a variety of materials, including powder samples and thin films.
Uses of Infrared Spectrophotometers
Infrared spectrophotometers (IR) are used in a wide range of fields, including pharmaceuticals, agriculture, biology, gas analysis, and forensics, where organic compounds are handled. The technique is used for qualitative and quantitative analysis of substances.
One of its main applications is the partial structure determination of compounds. This is based on the fact that each functional group has a unique absorption, and each peak is detected in a nearly constant wave number range (characteristic absorption band).
Since IR spectra are unique to a substance, they can also be used to identify unknown samples by comparing the measured spectrum with that of a standard sample. Infrared spectrophotometers, which can locally irradiate infrared light, can be used to measure minute amounts of samples and identify foreign substances in materials.
Principle of Infrared Spectrophotometers
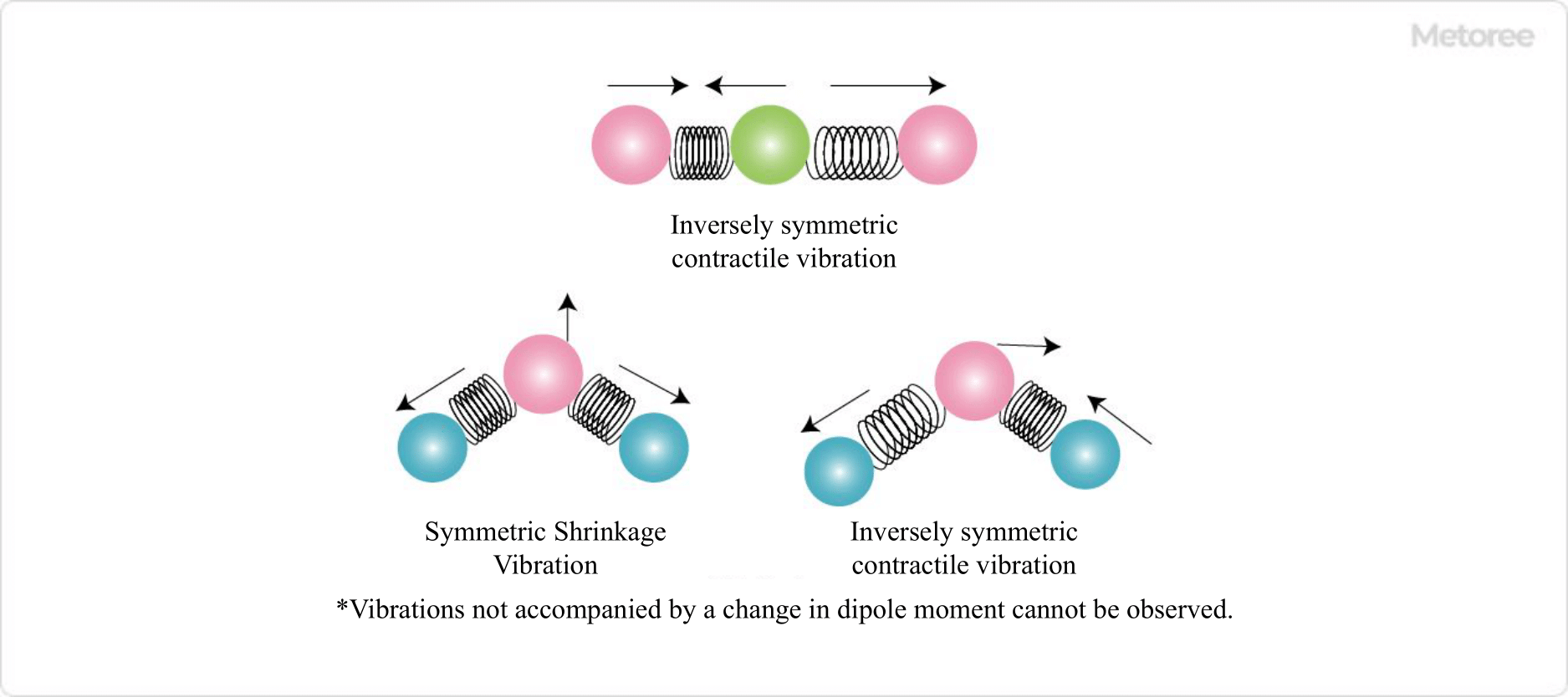
Figure 2. Examples of molecular vibrations observed by infrared absorption
The technique used in infrared spectrophotometers is called infrared spectroscopy (IR). When a substance is irradiated with infrared light (2500-25000 nm), absorption occurs based on the vibration and rotation of molecules.
At this time, the bonds connecting atoms in a molecule show different stretching and contraction depending on the type of bond, and as a result, the absorption spectrum also differs depending on the type of bond. This is the reason why IR is suitable for structure determination of functional groups. The type of functional group can be determined by examining the wave number of the absorbed IR radiation.
The detector measures the degree to which the IR radiation is reduced from the irradiated IR radiation by absorption (or reflection) by the sample. The resulting IR spectrum (infrared absorption spectrum) has the wave number of the irradiated infrared light (unit: cm-1, read: Kaiser) on the horizontal axis and the transmittance %T on the vertical axis.
Types of Infrared Spectrophotometers
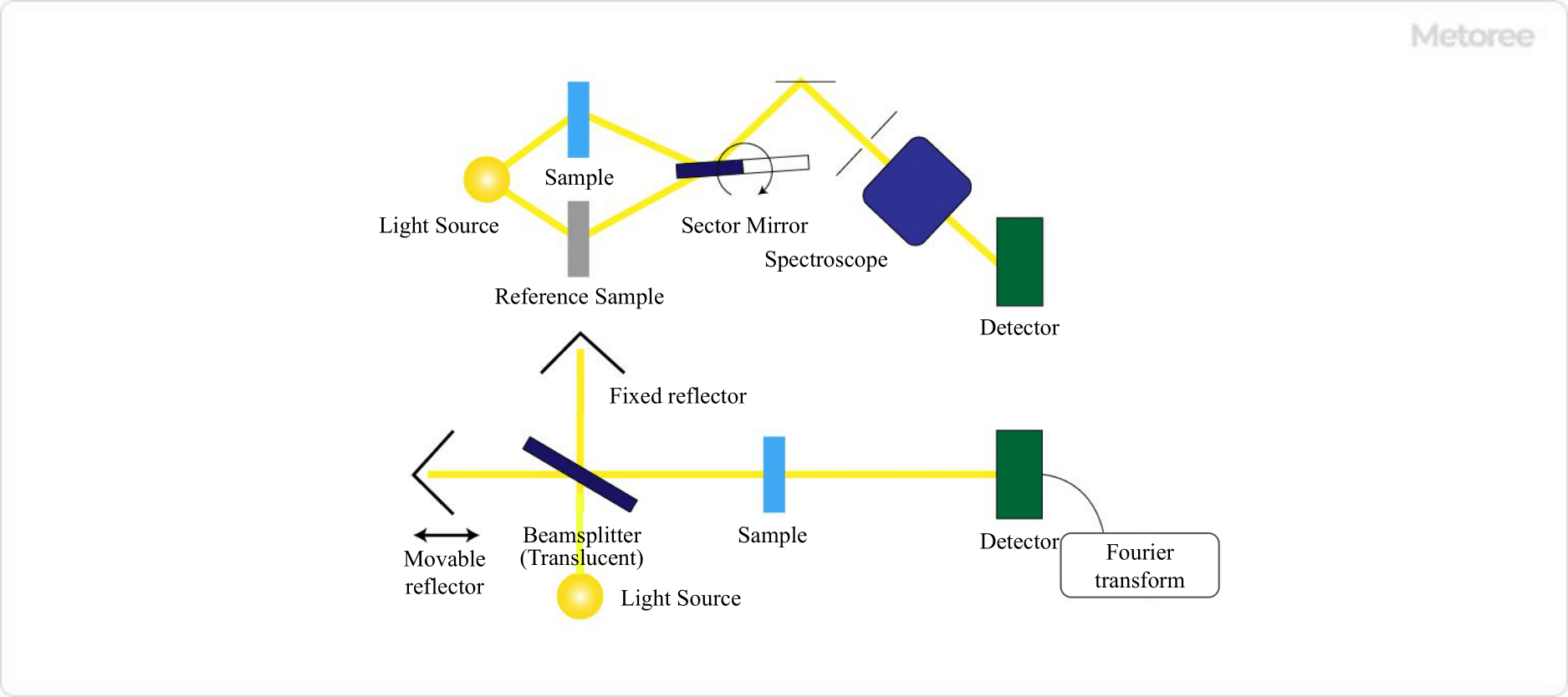
Figure 3. Schematic of dispersive IR (top) and FT-IR (bottom)
There are two types of infrared spectrophotometers: dispersive type and Fourier transform type (Fourier transform Infrared spectrophotometer FT-IR).
1. Dispersive Type
In the dispersive type, a diffraction grating is used in the spectrometer to disperse the light after it has passed through the sample, and each wavelength is detected by the detector sequentially.
2. Fourier Transform Type (FT-IR)
In the Fourier transform type, an interferometer is used to create interference waves, which are then irradiated onto the sample. After simultaneously detecting all wavelengths in a non-dispersive manner, the Fourier transform is performed on a computer to calculate each wavelength component.
It is possible to measure at all wavelengths at once, making measurements quick and easy. Because of its superior sensitivity and resolution, the Fourier transform type is currently the mainstream infrared spectroscopy method.
The advantages of the Fourier transform type (FT-IR) over the dispersive type include the following four points:
Simultaneous Detection of Multiple Wavelengths
In the Fourier transform type, IR spectra are obtained by moving a moving mirror. It is not necessary to move the diffraction grating to scan multiple wavelengths, as is the case with the dispersive type and thus enables high-speed measurement.
FT-IR is far more time-efficient when there are many objects to be measured or when noise is to be reduced by using a large integration time. In addition, since multiple wavelengths can be measured at the same time, there is an advantage in that there is less temporal variation in each wavelength (reduction of temperature drift of the measurement device).
Improvement of SNR
While dispersive IR uses a slit, FT-IR does not use a slit, and the energy reaching the detector is larger, resulting in improved SNR.
High Wave Number Resolution
Unlike dispersive IR, which requires a narrower slit to measure spectra with high wave number resolution, the wave number resolution of FT-IR can be easily increased by extending the moving mirror travel distance.
Possible to Expand the Measurement Wave Number Range
The wave number range can be extended from far infrared to visible by replacing the light source, beam splitter, detector, and window plate.
Other Information on Infrared Spectrophotometers
Preparation of Measurement Samples
Most compound identification using infrared spectrophotometers is done by the transmission method. The transmission method includes the use of powdered samples sandwiched between KBr plates (KBr plate method) or powdered samples mixed with KBr powder and solidified into a tablet form (KBr tablet method).
The sample is irradiated with infrared light, and the transmitted infrared light is analyzed. For samples with hygroscopic properties, powdered samples, and liquid paraffin are kneaded together to form a paste that is applied to a window plate (Nujol method). Samples on thin films, such as polymer compounds, can be directly irradiated with infrared light for measurement because infrared light penetrates the sample.
Note that there are some absorbers that cannot be analyzed depending on the preparation method. For example, in the KBr tablet method, it is difficult to evaluate the absorption band of the OH group due to the effect of moisture absorption of KBr, and in the Nujol method, the corresponding absorbent cannot be measured because of the absorption of liquid paraffin.