What Is Zinc?
 Zinc (element symbol: Zn) is a transition metal element with atomic number 30. Zinc alone has an atomic weight of 65.38, a density of 7.12 g/cm3, a melting point of 419.5°C, and a boiling point of 907°C. It is a silvery-white metal with a bluish tint. It is an amphoteric metallic element, soluble in both acids and alkalis.
Zinc (element symbol: Zn) is a transition metal element with atomic number 30. Zinc alone has an atomic weight of 65.38, a density of 7.12 g/cm3, a melting point of 419.5°C, and a boiling point of 907°C. It is a silvery-white metal with a bluish tint. It is an amphoteric metallic element, soluble in both acids and alkalis.
One of its characteristics is that it has a relatively high ionization tendency, and this property can be used for battery electrodes and zinc plating. It is also an essential trace element in living organisms, and plays an important role in maintaining normal sense of taste and in constituting enzymes that regulate metabolism.
Chemical Properties of Zinc
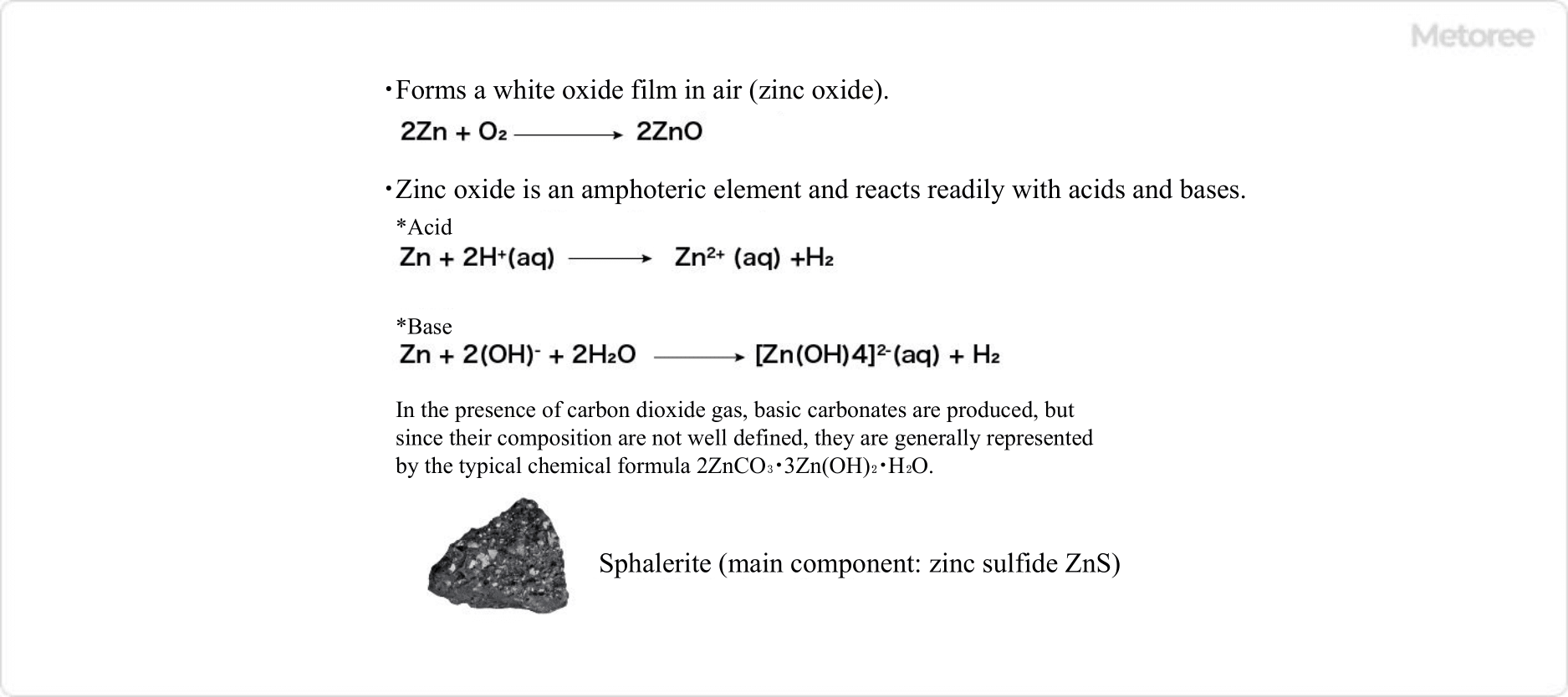
Figure 2. Chemical properties of zinc and image of zinc ore
Chemical Reactions
When left in the air, zinc on its own gradually forms an oxide film and loses its metallic surface luster. It is insoluble in water, but dissolves in non-oxidizing acids by emitting hydrogen gas, and dissolves in alkaline solutions to form zinc acid-alkali complex salts.
For example, when reacting with hydrochloric acid, zinc chloride and hydrogen are the products. When reacting with sodium hydroxide, tetrahydroxozinc(II) ion and hydrogen are formed. In addition, when carbon dioxide is present, basic carbonates are formed.
Compounds of Zinc
Natural zinc ores include sphalerite (blende or sphalerite, chemical formula: ZnS), anorthite (hemimorphite or calamine, chemical formula: Zn4Si2O7(OH)2・H2O), smithsonite (chemical formula: ZnCO3), and zincite (chemical formula: ZnO). Zinc ore is basically produced in a state in which it is combined with other elements such as sulfur, silicon, and oxygen.
Sphalerite is considered to be a particularly important ore as a source of zinc. Sphalerite may also contain rare metals such as indium and gallium.
Uses of Zinc
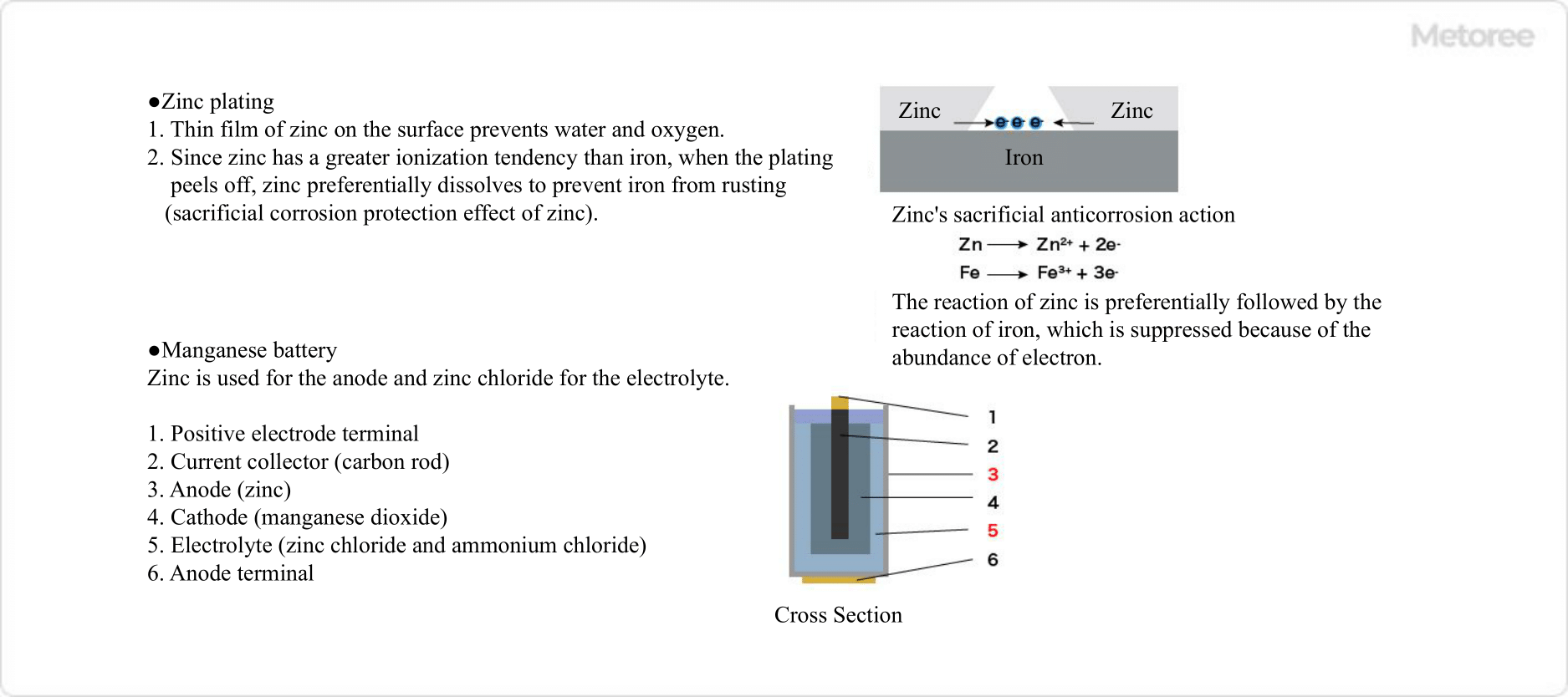
Figure 3. Examples of industrial applications of zinc
- Alloys
Uses for alloys include brass (copper and zinc), nickel silver (copper, zinc and nickel) and die castings. - Zinc plating
Zinc’s property of having a greater ionization tendency than iron is utilized in zinc plating. When zinc plating is applied to the surface of steel materials, a thin film of zinc on the surface prevents water and oxygen from entering the material, thus inhibiting the formation of iron rust.
In addition, since the ionization tendency of zinc is greater than that of the internal steel, even if the steel is exposed due to scratches, etc., the surface zinc will preferentially dissolve to protect the internal steel. Zinc plating is used in various fields such as automotive parts, electrical products, computers, and building materials. - Zinc Rich Paint
Paints containing 70% to 95% zinc powder are called zinc-rich paints and are used for rust-preventive coatings. In addition to direct coating, it can also be used as a repair agent for hot-dip galvanizing.
Epoxy resins are generally used for organic zinc rich paints and alkyl silicates for inorganic zinc rich paints as spreading agents to form the coating film. - Battery Electrodes and Electrolyte
In manganese dry cell batteries, zinc is used for the negative electrode and zinc chloride for the electrolyte. Other uses for zinc chloride include activated carbon, dyes, and agrochemical production. - Zinc Oxide
The white powder of zinc oxide is also used in pigments, sunscreens, and pharmaceuticals. It is also widely used in cosmetics, especially as a substitute for lead, which was once used in face powder and is highly toxic. Zinc is considered to be extremely low in toxicity compared to lead. - Zinc Sulfate
Zinc sulfate is used as a solution to coagulate liquid rayon in the rayon manufacturing process. It is also used as an additive in eye drops and is sometimes added to powdered formulas for child care, pets, and livestock to strengthen their mineral content. In addition, it is used to prevent damage to crops from pesticides such as Bordeaux solution, which is a fungicide.
2. Zinc in the Human Body and Foods
Zinc is present in the adult body in amounts of approximately 2,000 mg, and is mostly distributed in various muscles, bones, skin, liver, and brain. It is involved in various reactions in the body as a structural component of zinc-containing enzymes (DNA polymerase, RNA polymerase, alcohol dehydrogenase, carbonic anhydrase, etc.), which have metabolic regulating effects. Typical roles include DNA synthesis, protein synthesis, removal of reactive oxygen species, and maintenance of normal taste.
According to the 2020 Dietary Reference Intakes, the recommended intake of zinc is set at about 11 mg/day for adult males and 8 mg/day for adult females. A deficiency of zinc can cause symptoms such as dermatitis, taste disorders, and immune dysfunction. In children, it has also been noted that growth retardation may occur.
Zinc is abundant in fish, shellfish, and meat, and specific foods include oysters, pork liver, and lean beef. It is also found in soybean flour and nuts.
When zinc is taken together with citric acid and vitamin C, its intake efficiency is said to increase. On the other hand, phytic acid, which is abundant in rice bran and brown rice, inhibits the absorption of zinc, so it is necessary to consider food combinations.
3. Zinc Supplements
Zinc is available commercially as a stand-alone supplement, as well as in combination with other ingredients such as multivitamins and minerals. Zinc in supplements takes a variety of salt forms, including zinc gluconate and zinc sulfate.
Supplements are generally taken with water during or after meals. Coffee, tea, and other beverages containing caffeine or tannins may bind them to the nutrients and inhibit their absorption.
Excessive consumption of zinc may also cause copper deficiency, nausea, vomiting, stomach problems, and immune disorders.