What Is Hydrogel?
Hydrogel is a general term for a solid such as a polymer that absorbs water and swells to form a non-flowable gel.
For example, when polymer chains such as polysaccharides and gelatin are cross-linked to form a three-dimensional network structure, the network structure contains a large amount of water and becomes a swollen body that cannot be dissolved in water. Examples include konjac, agar, and jelly.
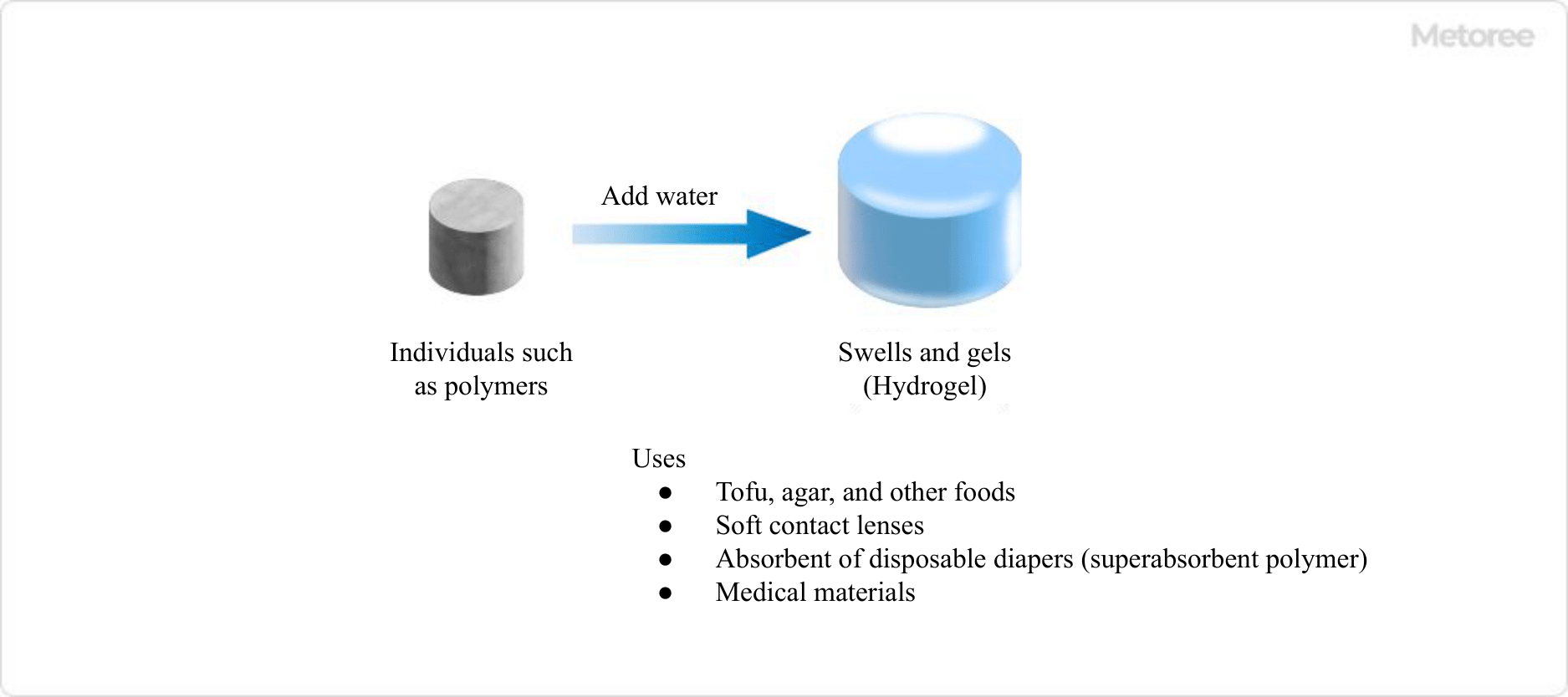
Figure 1. Image of hydrogel
Uses of Hydrogel
Hydrogel is found in foods such as tofu and agar, and is also used in soft contact lenses and diaper absorbents (superabsorbent polymers). Because its composition is similar to that of biological soft tissue, its use as a medical material has been explored in recent years, but the loss of properties due to absorption of water in the body remains an issue to be resolved.
Examples include the use as artificial cartilage and artificial intervertebral discs, as a material that releases drugs slowly, and as a scaffold material for cells in the field of regenerative medicine.
After culturing cells on Hydrogel, only the gel is dissolved by a reducing agent to create cell sheets with cells attached to each other, which can then be applied to damaged areas for treatment.
Principles and Properties of Hydrogel
1. Physical Gel and Chemical Gel
Hydrogel is classified into physical gel and chemical gel according to the cross-linking method.
- Physical gel
Cross-linked by hydrogen bonding, ionic bonding, coordination bonding, etc. - Chemical gels
Cross-linked by covalent bonding
As specific examples, items such as agar and gelatin, which undergo a reversible sol-gel transition when heated, are physical gels, while chemically stable items such as superabsorbent polymers used in disposable diapers and soft contact lenses are chemical gels.
2. Examples of Gelation
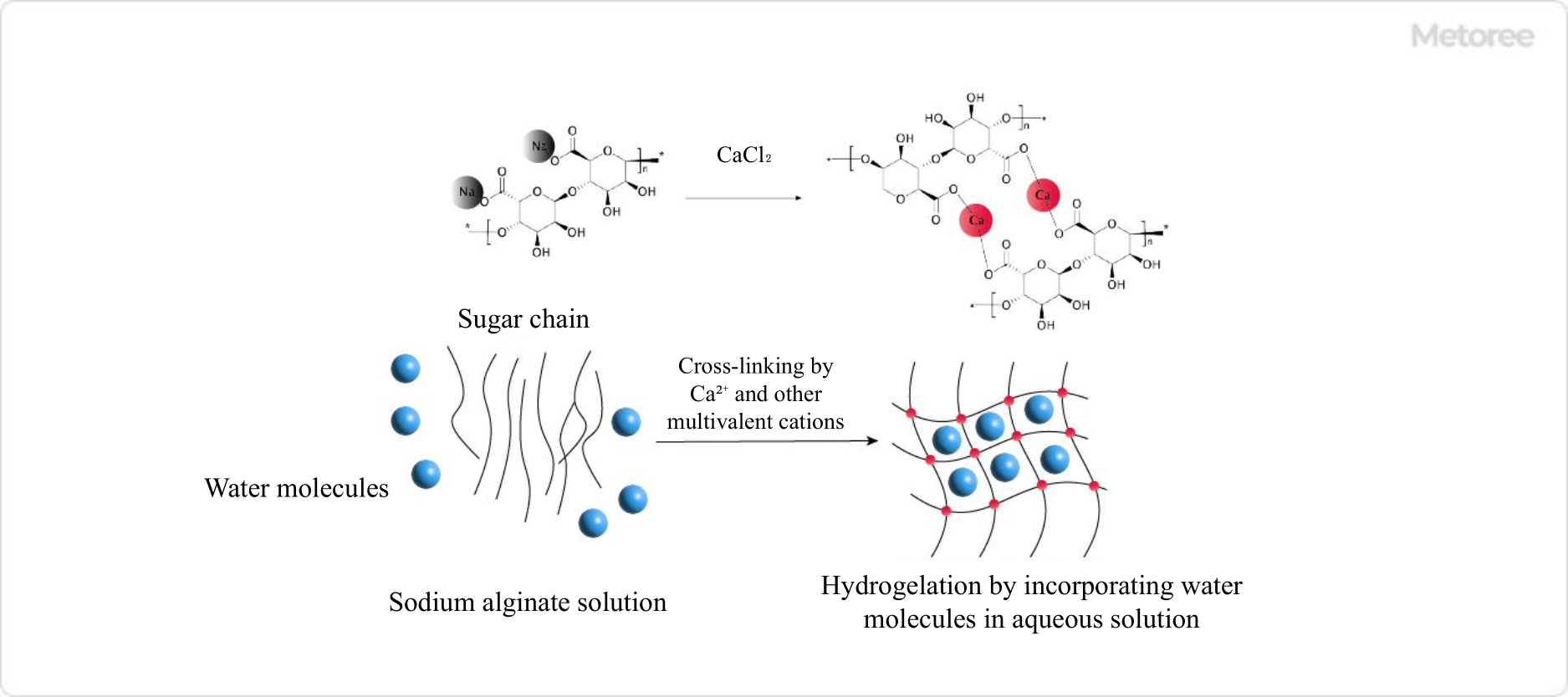
Figure 2. Example of gelation of sodium alginate
One well-known example is alginic acid, a natural polymer. The sodium salt of alginate is water soluble, but when a multivalent cation such as Ca2+ is added, ionic cross-linking occurs instantly. In this process, the solvent water is incorporated into the mesh structure of the cross-links, resulting in gelation (Hydrogel).
Types of Hydrogel
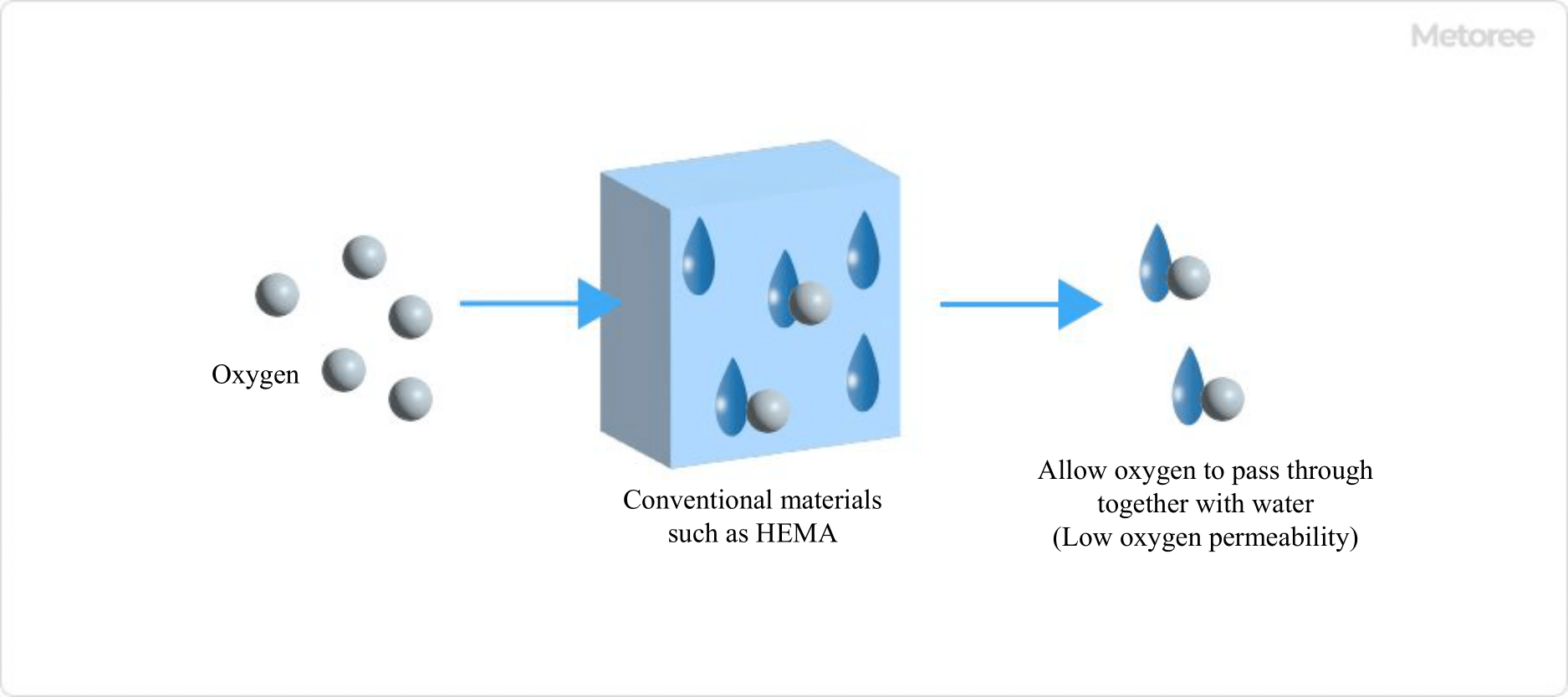
Figure 3. Conventional materials such as HEMA
HEMA (Hema: hydroxyethyl methacrylate) has been used in conventional soft contact lenses because it becomes soft when water is included. Since increasing the water content increases oxygen permeability, attempts have been made to increase the water content and to reduce the thickness of the lens. However, since a high water content rate causes water to evaporate more easily, the eyes tend to dry out during wear, and technological development has been considered to be limited.
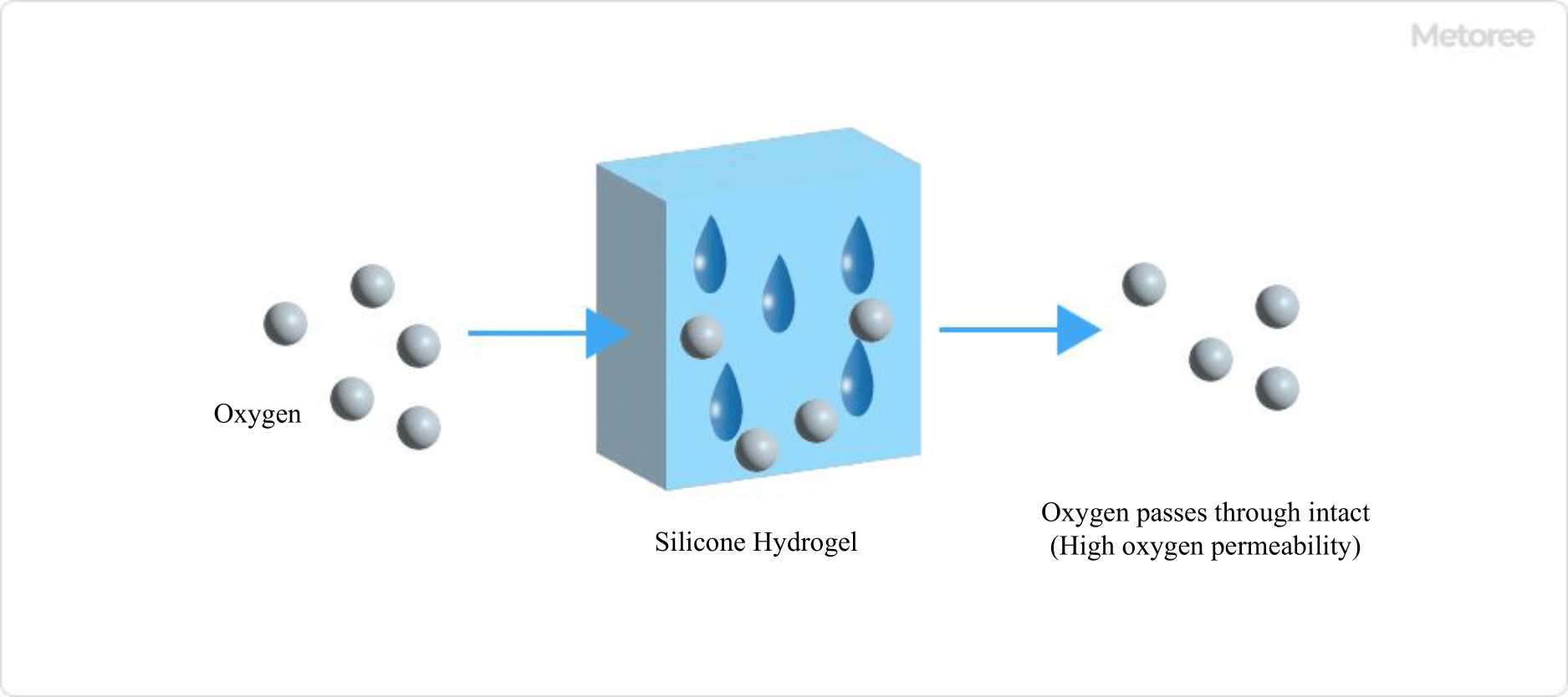
Figure 4. The case of silicone hydrogel
Therefore, silicone hydrogel has been attracting attention in recent years as a new material that solves the HEMA issue. Silicone hydrogel is a material that exhibits high oxygen permeability despite its low water content. Since oxygen passes directly through the contact lens, it can deliver a large amount of oxygen without depending on the amount of water in the lens, which has the advantage of reducing the burden on the eyes.
It is expected to reduce the decrease in corneal endothelial cells in the cornea, which has been a problem with contact lenses in the past. Another advantage is that the low water content prevents the eyes from drying out while wearing the lenses. Furthermore, silicone hydrogel material is less likely to be contaminated by proteins contained in tears.
However, because it is highly lipophilic, once oil gets on it, it is difficult to remove. Therefore, care must be taken to prevent oil from adhering to eye makeup. The challenge is that it is a harder material than HEMA due to its low water content, and technological development is underway to improve its wearing comfort.