What Is Chloric Acid?
Chloric acid is an oxoacid of chlorine with the chemical formula HClO3.
The CAS number is 7790-93-4. Chloric acid cannot be isolated in its free acid form and is only available as an aqueous solution.
Uses of Chloric Acid
The use of sodium chlorate, a compound related to chloric acid, was banned in the EU in 2009 for herbicidal applications due to its environmental impact.
1. Oxidizing and Bleaching Agents
Aqueous solutions of chloric acid are potent oxidizers and strong acids. They are used industrially as a bleaching agent for pulp and various materials, due to their effectiveness.
These solutions also serve as a raw material for chloric acid salts (such as sodium chlorate, potassium chlorate, ammonium chlorate, zinc chlorate, etc.), dissolving many metals along with their oxides, hydroxides, and carbonates.
2. Gunpowder and Explosives
Chloric acid salts have been historically used as raw materials for gunpowder and explosives. Sodium chlorate, with a purity of 98%, was previously utilized as an agricultural chemical, but its potential for explosion and misuse as an illegal explosive led to social issues.
Therefore, since the 1970s, alternatives like sodium carbonate have become more mainstream.
Properties of Chloric Acid
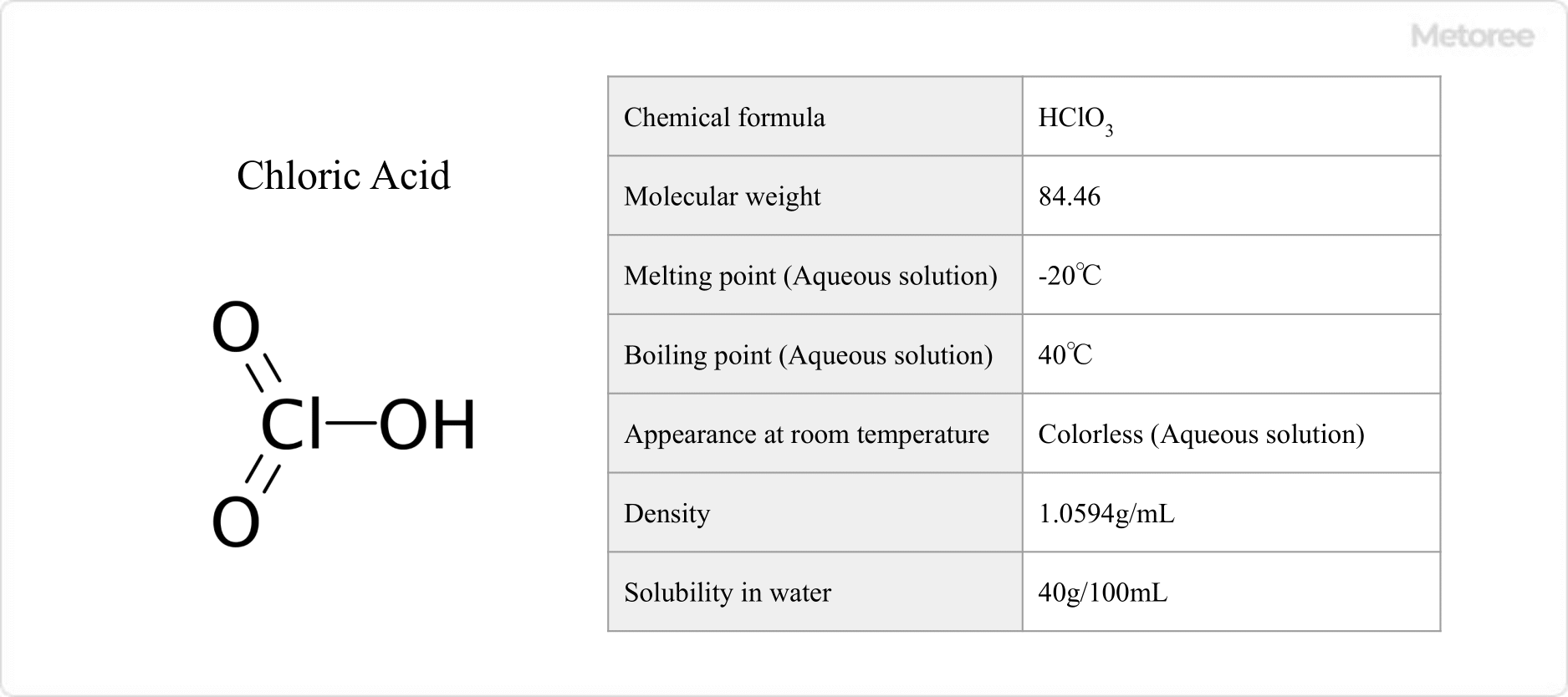
Figure 1. Basic Information on Chloric Acid
Chloric acid has a molecular weight of 84.46, melts at -20°C, and boils at 40°C in solutions of less than 10% concentration. Its aqueous solutions are colorless, with a 10% solution having a density of 1.0594 g/mL. Solubility in water is 40 g/100 mL (at 20°C).
A cold aqueous solution of chloric acid is stable up to about 30% concentration, which can be concentrated to 40% by careful decompression.
Types of Chloric Acid
Chloric acid is primarily available in the form of stable chlorates, such as sodium chlorate and potassium chlorate.
Free acid products are not available. It is important to differentiate between aqueous solutions of chloric acid and similarly named substances, such as hypochlorite and chlorite solutions, as they are distinct.
Other Information on Chloric Acid
1. Synthesis of Chloric Acid
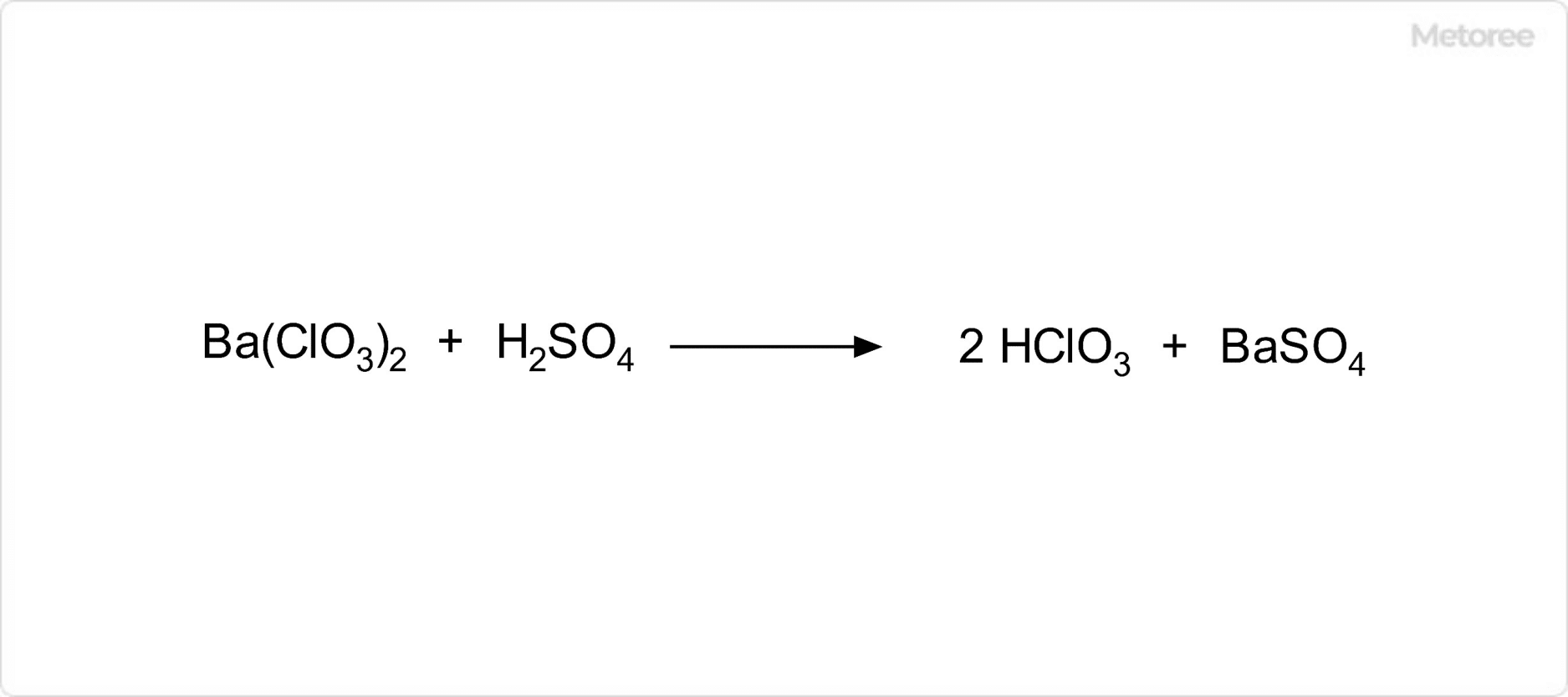
Figure 2. Synthesis of Chloric Acid
Chloric acid can be synthesized by reacting dilute sulfuric acid with an aqueous solution of barium chlorate, precipitating insoluble barium sulfate and forming chloric acid in the solution, which is then isolated by removing the barium sulfate.
2. Chemical Reaction of Chloric Acid
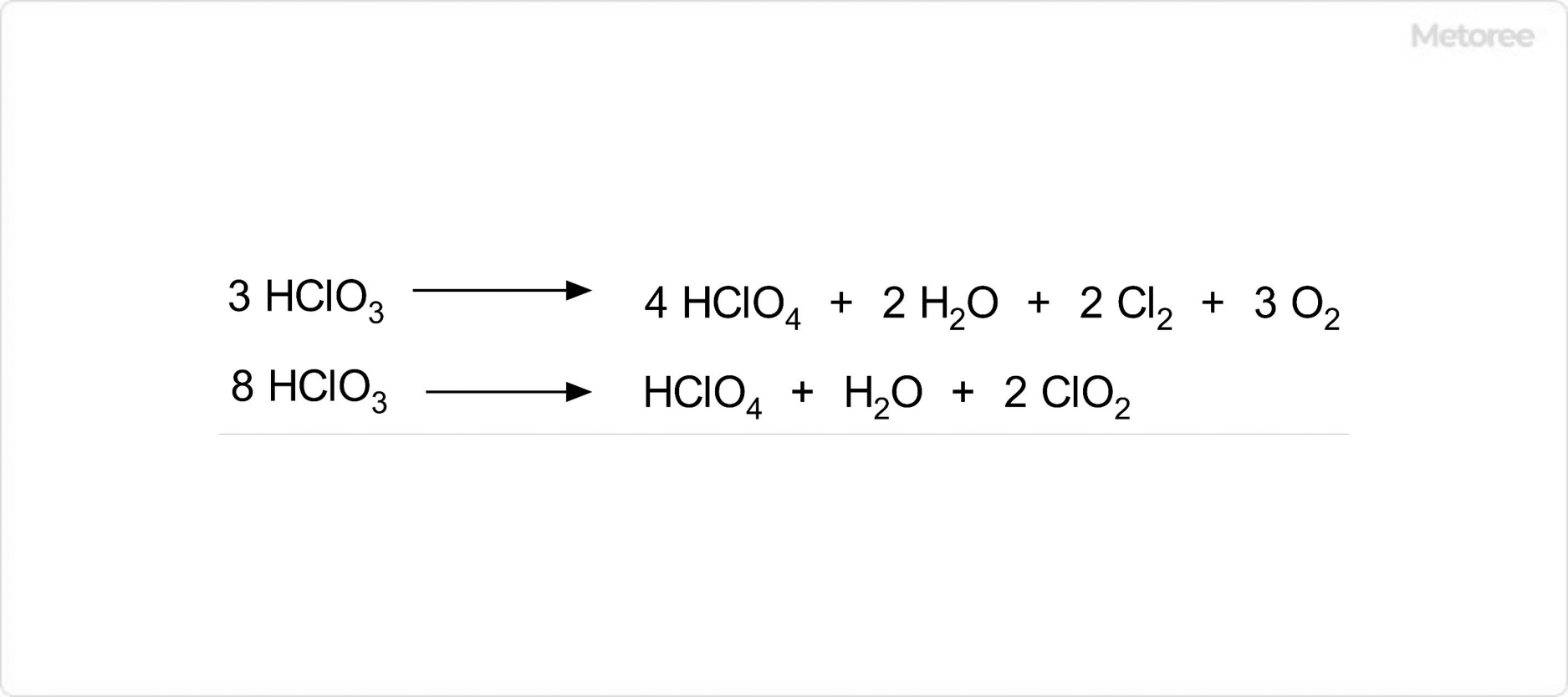
Figure 3. Decomposition Reaction of Chloric Acid
Under reduced pressure, chloric acid can be concentrated to about 40%. This process yields various chlorine compounds, including perchloric acid, chlorine dioxide, and chlorine. Chloric acid solutions can ignite organic materials on contact, so mixing with these materials, metal powders, and ammonia, which can form explosive mixtures, should be avoided.
3. Related Substances of Chloric Acid
Related substances of chloric acid include various unstable chlorates that require careful handling:
- Sodium Chlorate
- Potassium Chlorate
- Ammonium Chlorate
- Calcium Chlorate
- Barium Chlorate
- Zinc Chlorate
- Silver(I) Chlorate
4. Hazardous Properties of Aqueous Solutions of Chloric Acid
Aqueous solutions of chloric acid are classified by the GHS as follows. Proper safety measures, including local exhaust ventilation and personal protective equipment, should be used when handling these solutions:
- Oxidizing liquid: Category 1-2
- Corrosive to metals: Category 1