What Is Quinoxaline?
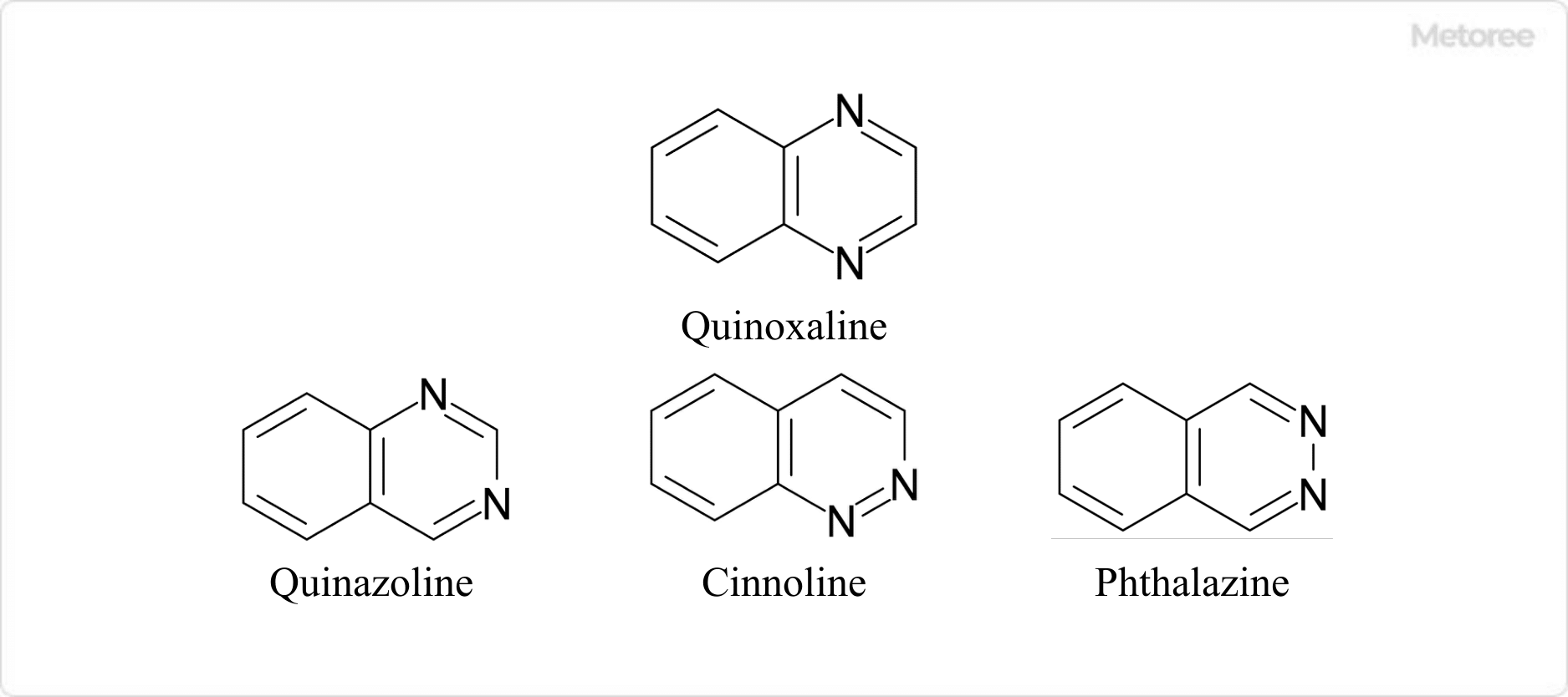
Figure 1. Quinoxaline and Its Isomers
Quinoxaline is a heterocyclic compound with the chemical formula C8H6N2, consisting of a benzene ring fused to a pyrazine ring. It is also known as benzopyrazine, and its CAS registration number is 91-19-0. Quinoxaline differs from its isomers — quinazoline, cynnoline, and phthalazine — and does not fall under any specific GHS classification.
It is not subject to regulation under the Industrial Safety and Health Law, the Labor Standards Law, the PRTR Law, or the Poisonous and Deleterious Substances Control Law.
Uses of Quinoxaline
Quinoxaline is widely used as a dye, but also as a functional material in applications such as photoreceptors for electrophotography. Additionally, it serves as an intermediate and starting material in the pharmaceutical and agrochemical industries. Compounds featuring the quinoxaline structure can inhibit enzyme activity by capturing metal ions involved in metabolic processes, leading to its use in fungicides, acaricides, and antibiotics such as ethinomycin, levomycin, and actinoleutin.
Properties of Quinoxaline
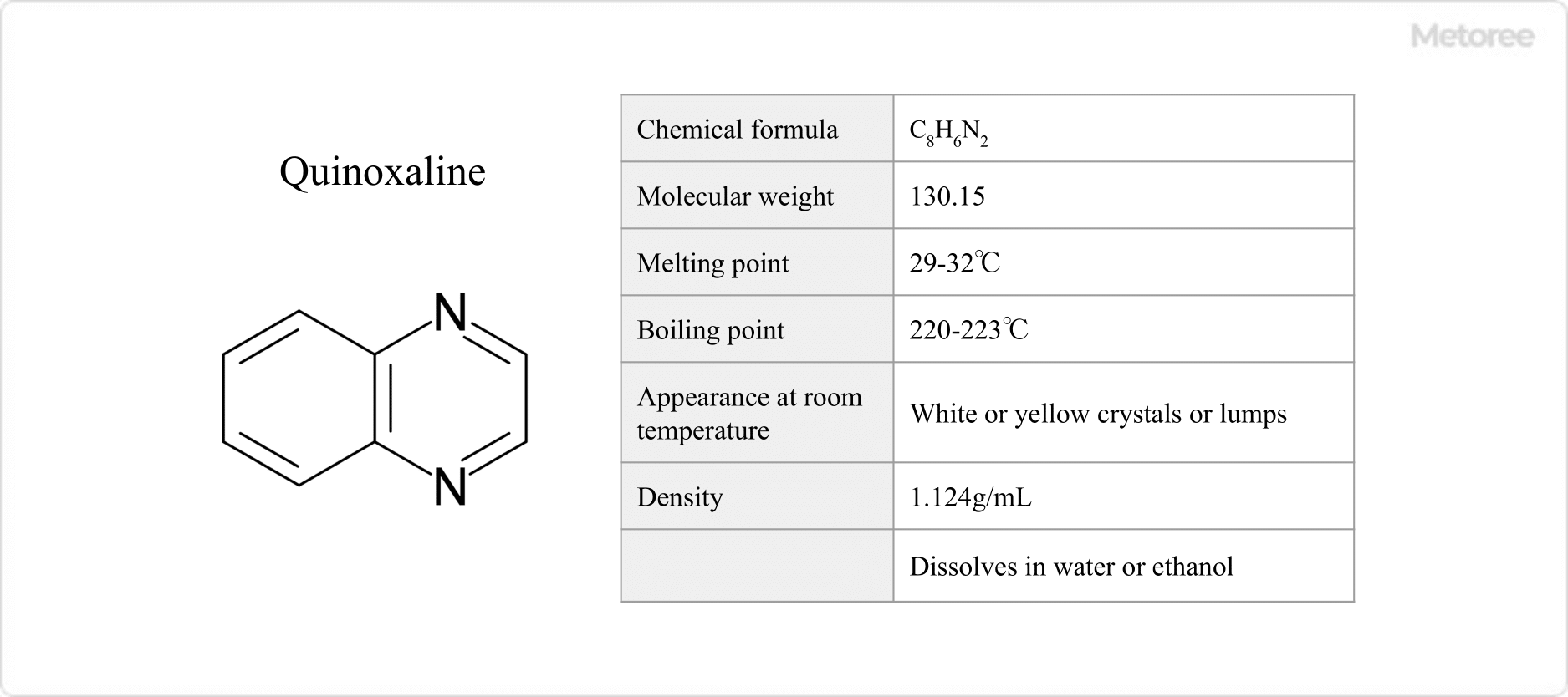
Figure 2. Basic Information on Quinoxaline
Quinoxaline has a molecular weight of 130.15, a melting point of 84-90 °F (29-32 °C), a boiling point of 428-433 °F (220-223 °C), and takes the form of a white or yellow crystal or mass at room temperature. It has a density of 1.124 g/mL and a flash point of 208 °F (98 °C). Quinoxaline is soluble in water and ethanol.
Types of Quinoxaline
Quinoxaline, primarily sold as a reagent for research and development, is available in quantities suited for laboratory use, such as 25g. Due to its low melting point, it is often stored at refrigerated temperatures of 32-50 °F (0-10 °C).
Other Information on Quinoxaline
1. Synthesis of Quinoxaline
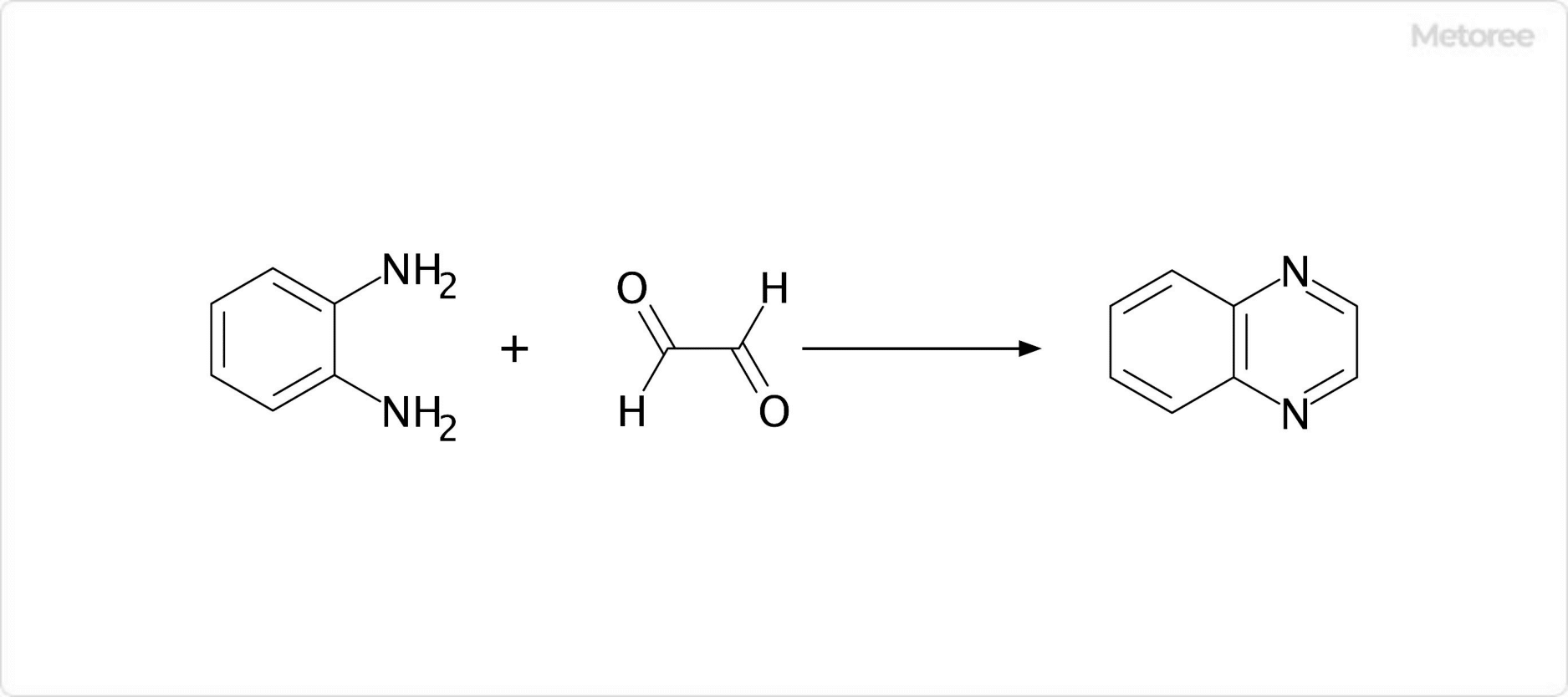
Figure 3. Example of Synthesis of Quinoxaline
Quinoxaline synthesis typically involves reacting o-diamines with diketones. Unsubstituted quinoxaline can be produced by reacting o-phenylenediamine with glyoxal. Other methods include the synthesis of quinoxaline derivatives from o-phenylenediamine and benzyl, using 2-iodoxybenzoic acid (IBX) as a catalyst. Various functional groups can be introduced into the quinoxaline structure using substituents such as α-keto acids, α-chloroketones, α-aldehyde alcohols, and α-ketone alcohols as diketones.
2. Reactivity of Quinoxaline
Quinoxaline is stable under normal storage conditions but is considered a flammable organic substance. If finely dispersed, a dust explosion may occur, although no hazardous interactions have been specifically identified.
3. Derivatives of Quinoxaline
Available derivatives of quinoxaline include quinoxaline-2,3-dithiol, quinoxaline-2,3-diol, quinoxaline-2-carboxyaldehyde, quinoxaline-5-ol, quinoxaline-6-methyl carboxylate, and carboxylic acid-6-amine, all sold for research and development purposes.
4. Precautions for Handling Quinoxaline
Although not classified under the GHS, quinoxaline should be handled with care due to potential skin and eye irritation risks. Personal protective equipment such as gloves, eye protection, and face protection is recommended. In case of skin or eye contact, rinse thoroughly with water. If wearing contact lenses, remove them if possible, and continue rinsing.