What Is Acetoacetic Acid?
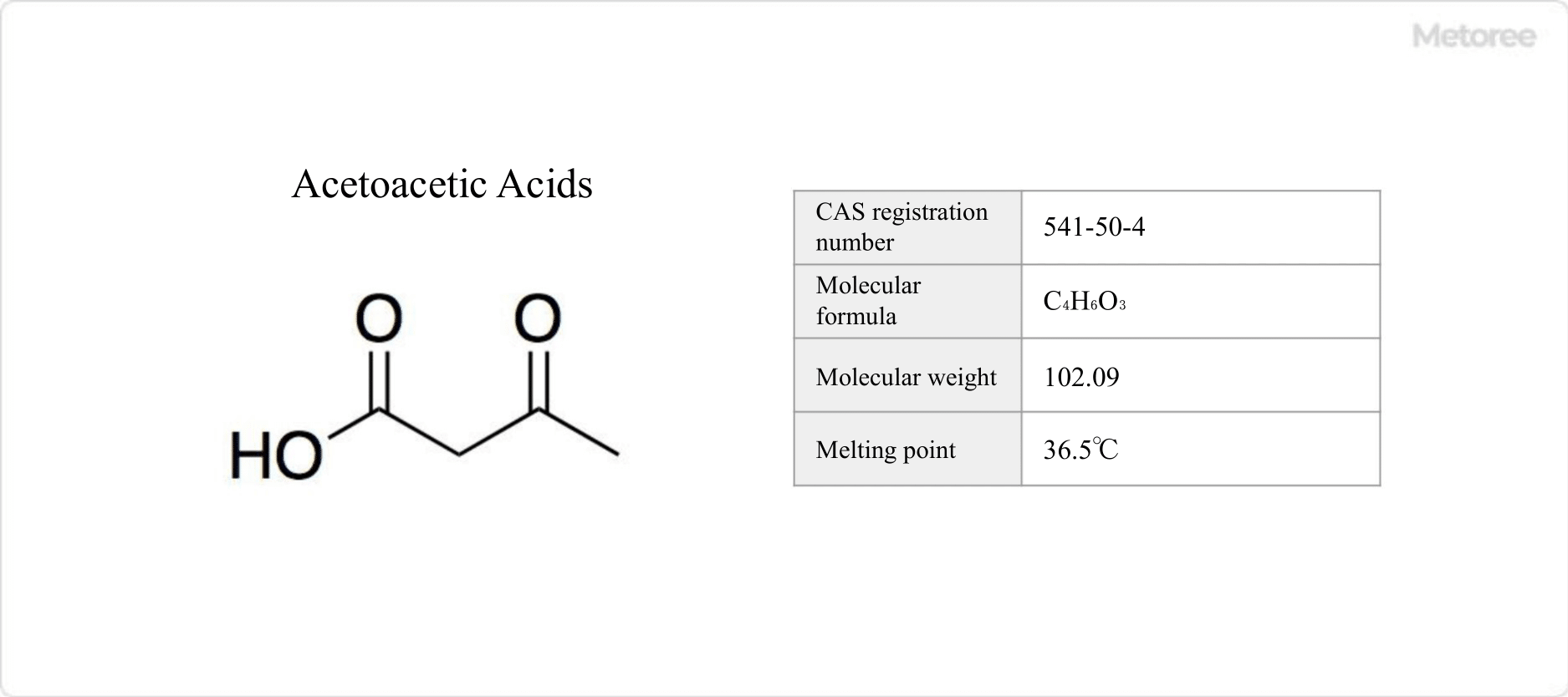
Figure 1. Basic information on acetoacetic acid
Acetoacetic acid, a carboxylic acid compound with the chemical formula C4H6O3, is also known as 3-oxobutanoic acid. Typically unstable, it is often used in the form of its esters, such as ethyl acetoacetate or methyl acetoacetate. Ethyl acetoacetic acid, commonly handled, is classified as a flammable liquid and eye irritant under the GHS classification and as a Class 4 hazardous material under the Fire Service Law, but is exempt from several other regulations.
Uses of Acetoacetic Acid
Acetoacetic acid is primarily used as a reaction intermediate in organic synthesis, obtained through the saponification of esters like methyl and ethyl acetoacetate. Biochemically, its concentration increases in the blood under certain conditions, such as in diabetics and during strenuous exercise while fasting. This increase is due to enhanced fatty acid breakdown, leading to the formation of acetoacetic acid, a condition known as ketosis, which can affect appetite and gastrointestinal function.
Properties of Acetoacetic Acid
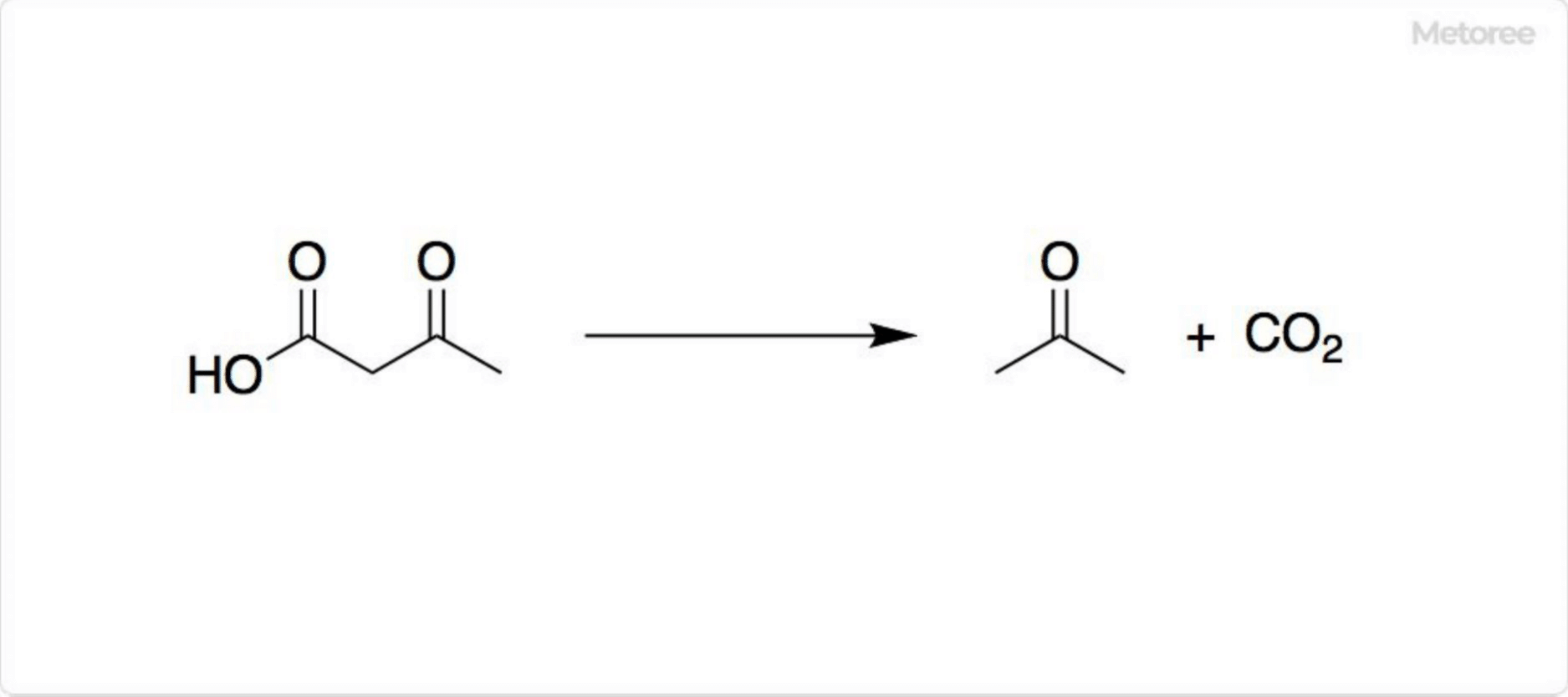
Figure 2. Reaction of acetoacetic acid
Acetoacetic acid melts at 97.7°F (36.5°C) and decomposes into acetone and carbon dioxide gas over time or when heated. Its acid form has a half-life of 140 minutes in water at 98.6°F (37°C), while its base form, as an anion, has a much slower half-life of 130 hours. It is a weak acid with a pKa of 3.58, similar to alkyl carboxylic acids.
Structure of Acetoacetic Acid
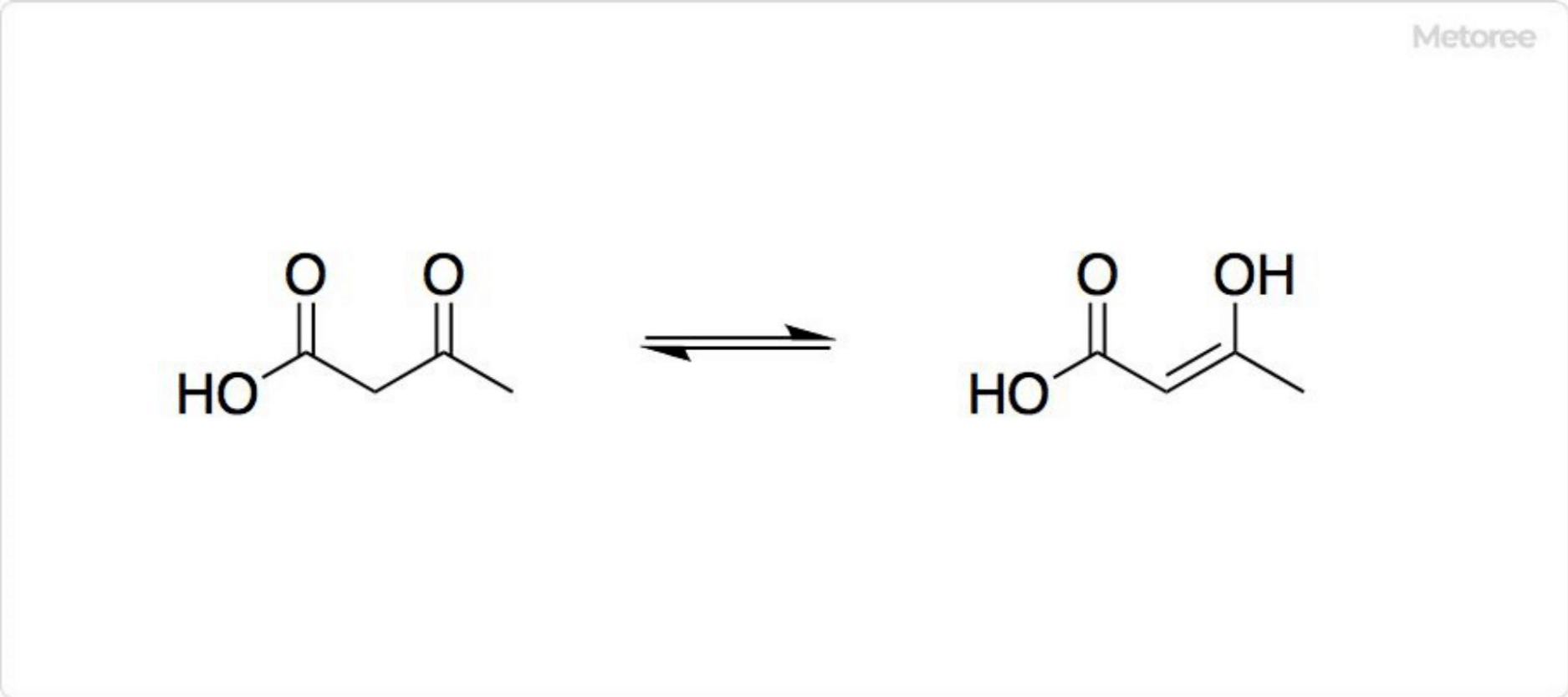
Figure 3. Structure of acetoacetic acid
As a β-keto acid, acetoacetic acid exhibits keto-enol tautomerisation, with the enol form partially stabilized by conjugation and intramolecular hydrogen bonding. This equilibrium is highly solvent dependent, with the keto form predominating in polar solvents like water (98%) and the enol form more prevalent in non-polar solvents (25-49%).
Other Information on Acetoacetic Acid
1. Synthesis of Acetoacetic Acid
Acetoacetic acid is produced by hydrolyzing diketene and can be synthesized from acetoacetic acid esters at 0 °C, decomposing rapidly into acetone and carbon dioxide for immediate use. Its esters are integral in acetoacetylation reactions, crucial for producing dyes such as arylide yellows and diarylides. Diketenes also react with alcohols and amines to yield corresponding acetoacetic acid derivatives.
2. Detection of Acetoacetic Acid
Acetoacetic acid is measured in the urine of diabetic patients or those on ketogenic or low-carbohydrate diets to confirm diabetic ketoacidosis. A dipstick coated with nitroprusside changes color in the presence of acetoacetate, the conjugate base of acetoacetic acid, from pink to purple, facilitating visual assessment.