What Is Adiponitirile?
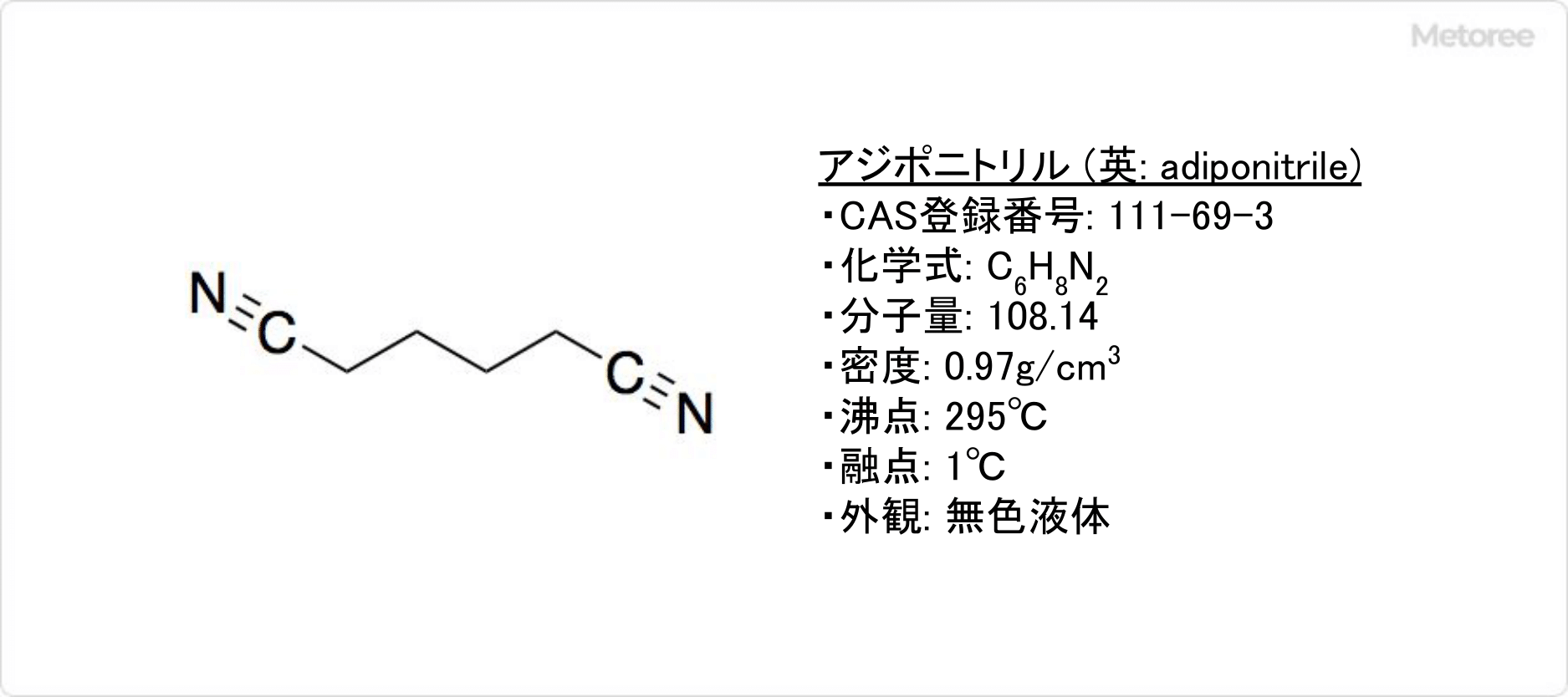 Adiponitirile is a type of dinitrile, a colorless liquid at room temperature.
Adiponitirile is a type of dinitrile, a colorless liquid at room temperature.
It is also known as “hexanedinitrile” or “1,4-dicyanobutane.” It is flammable and produces toxic gases. Like other cyanide compounds, it is highly poisonous.
Uses of Adiponitirile
Adiponitirile is an important intermediate used in the manufacture of Nylon 66. The majority of its uses are in the production of nylon. Nylon 66 has properties such as high strength, abrasion resistance, and electrical insulation, and is widely used in applications such as electronics, automotive, packaging, construction, and consumer goods.
In addition to being used as a raw material for nylon, Nylon 66 is also used as an intermediate in the manufacture of rust inhibitors and rubber vulcanization accelerators. It is often used as a structure of automobiles by compositing it with glass fiber and other materials.
Properties of Adiponitirile
Adiponitirile is soluble in water, methanol, ethanol, and chloroform. Its melting point is 33.8°F (1°C) and its boiling point is 563°F (295°C). Orally, like other cyanide compounds, it is deleterious, but respirationally, it is less hazardous due to its low vapor pressure.
Its molecular formula is C6H8N2 and its molecular weight is 108.14. It is an organic compound with two cyano groups, and its differential formula is NC(CH2)4CN, with a density of 0.97 g/cm3.
Other Information on Adiponitirile
1. Examples of Adiponitirile Synthesis
Generally, adiponitirile can be obtained by dehydrating adipoamide using a catalyst, such as vanadium pentoxide. An industrialized method is the hydrocyanation of butadiene.
Acrylonitrile is also obtained by electrolytic dimerization reduction of acrylonitrile, which is produced by ammoxidation of propene.
2. Details of the Synthesis of Adiponitirile
Specifically, ammonia reacts with adipic acid produced by the oxidation of cyclohexane. The resulting ammonium adipate can be obtained by dehydration using a phosphoric acid catalyst.
It can also be synthesized by hydrocyanation of butadiene. The reaction of cyanide with butadiene in the gas phase over a copper-magnesium chromite catalyst yields 3-pentenenitrile and 4-pentenenitrile as main products.
Adiponitirile can be obtained by further reacting these products with hydrogen cyanide acid in the liquid phase using a nickel complex catalyst.
3. Reaction of Adiponitirile
Hydrogenation of adiponitirile with nickel or other catalyst yields hexamethylene diamine. The process of hydrolysis will yield adipic acid. Nylon 66 is produced by condensation polymerization of adipic acid with hexamethylene diamine produced from adiponitirile.
4. Adiponitirile as a Synthetic Intermediate for Nylon
Adiponitirile is an important compound as an intermediate in the synthesis of Nylon 66. Both hexamethylenediamine and adipic acid, which are necessary to obtain Nylon 66, can be synthesized from adiponitirile.