What Is a Spectral Sensor?
The Spectral Sensor is a generic term for analytical equipment that can examine the composition and properties of a substance by measuring the light emitted or absorbed by the substance.
The device mainly consists of a light source, a Spectral Sensor, a sample, and a detector. There are various types of Spectral Sensors depending on the type of light source used and the mechanism of the device.
Specifically, there are UV-visible spectrophotometers (UV-Vis), Infrared Spectrophotometer (IR), Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES), Atomic Absorption Spectrometer (AAS), X-ray Fluorescence Spectrometer (XRF), X-ray Photoelectron Spectrometer (XPS), and others. Since the analysis capabilities of each instrument differ, it is necessary to select the one that best suits your purpose.
Uses of Spectral Sensors
Spectral Sensors are used in a variety of fields. Below are some typical applications. These are only a few examples; Spectral Sensors are used in a wide range of fields.
1. Chemistry And Biochemistry
Quality control of synthesized chemicals by checking their molecular structures, reaction rates, and impurity content, structural analysis of proteins and DNA, and measurement of enzyme reactions are examples.
2. Environmental Science
Detection and analysis of contaminants in water and air.
3. Medical and Pharmaceutical Sciences
Mass measurement of drugs, measurement of components in blood, diagnosis of diseases, etc.
4. Food Industry
Quantitative analysis of nutritional components and additives in food, quality control, composition analysis of materials, measurement of surface properties, and research on oxidation reactions.
Principle of the Spectral Sensor
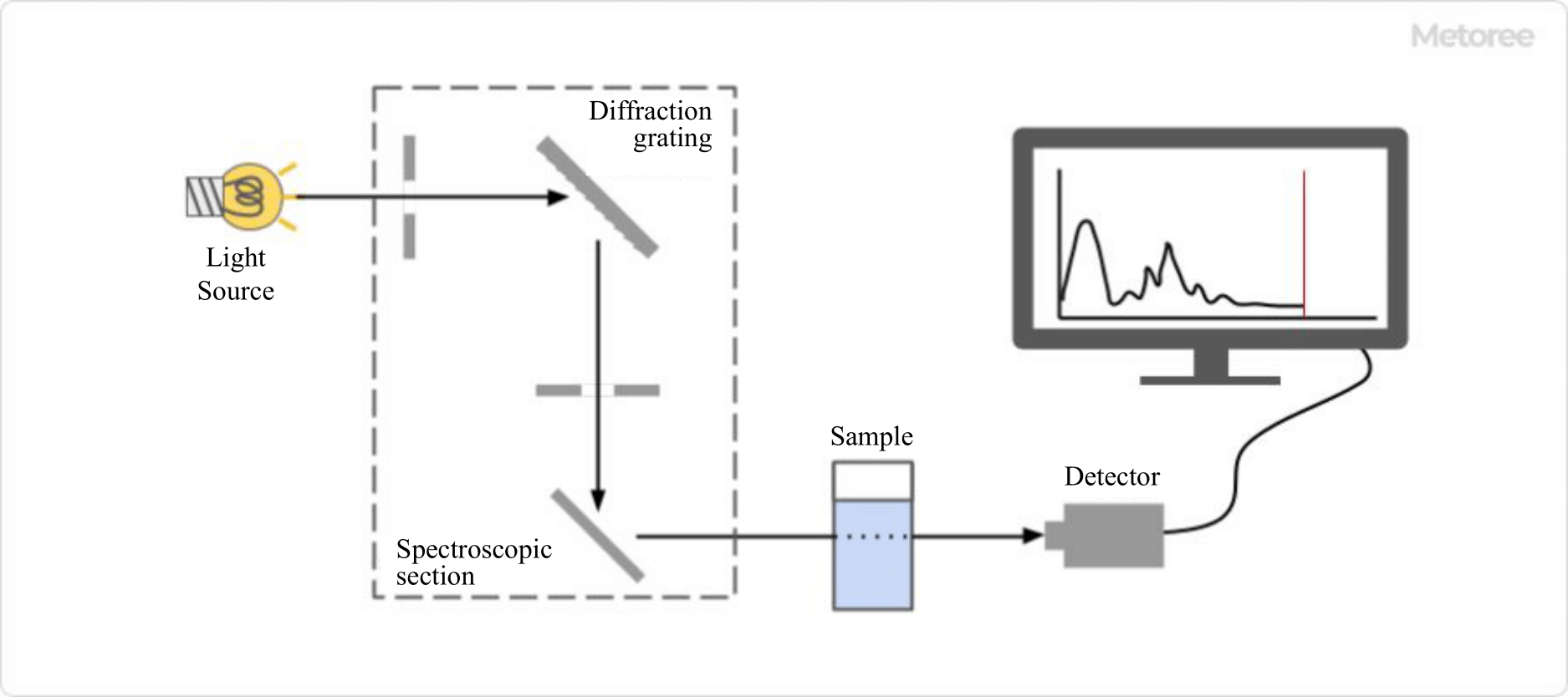
Figure1. Structure of a spectroscopic sensor
Spectral Sensors are used to identify and quantify substances in a sample by irradiating the sample with some kind of light and analyzing the light absorbed, reflected, or emitted from the sample. The results of the analysis are output as a waveform diagram called a spectrum.
By analyzing this spectral data, it is possible to perform, for example, qualitative and quantitative analysis of samples, evaluation of molecular structures, and evaluation of material properties. The measurement principle differs from one instrument to another, and the measurement principles of the six representative instruments mentioned above are briefly described below.
1. UV-Visible Spectrophotometer
When a sample is irradiated with light up to UV/visible wavelengths, the light is absorbed or reflected by the substances contained in the sample. By measuring the intensity of light absorbed or transmitted at each wavelength of incident light, the molecular structure of the components contained in the sample can be determined and quantitatively analyzed.
2. Infrared Spectrophotometer
When a sample is irradiated with infrared light, the sample absorbs or reflects the infrared light. The infrared radiation absorbed or reflected depends on the type of compound in the sample and the state of bonding. By dividing the infrared light into different wavelengths with a Spectral Sensor and measuring the light intensity with a detector, the type and bonding state of the compounds in the sample can be determined.
3. Inductively Coupled Plasma Atomic Emission Spectral Sensor
A sample is introduced into a flame called “plasma,” which is generated by burning a substance at a high temperature, and the luminescence is observed to determine the composition of the substance. When a sample is placed in the plasma, it is broken down into atoms and ions.
During this process, the atoms and ions in the plasma absorb energy and emit light when they release it. This light emission consists of light of various wavelengths, and by measuring the intensity and wavelength of the light, the components in the sample can be determined.
4. Atomic Absorption Spectral Sensor
Light emitted from a special light source is shone on a sample. Since elements absorb light at wavelengths specific to the element, the amount of the element in the sample can be determined by measuring the intensity of the absorbed light at each wavelength.
5. X-ray Fluorescence Analyzer
When X-rays strike a sample, the elements in the sample absorb energy and emit it, producing X-rays of fluorescence.
Since the energy of this X-ray fluorescence differs depending on the type of element, the energy of the X-ray fluorescence can be measured to determine which elements are contained in the sample.
6. X-ray Photoelectron Spectral Sensor
When a solid surface is exposed to X-rays, atoms and molecules are ionized, and electrons are emitted as a result of the ionization. The emitted electrons have different energies depending on the element and its chemical state.
By varying the energy of the X-rays, it is possible to examine the surface of a sample at various depths.
Types of Spectral Sensors
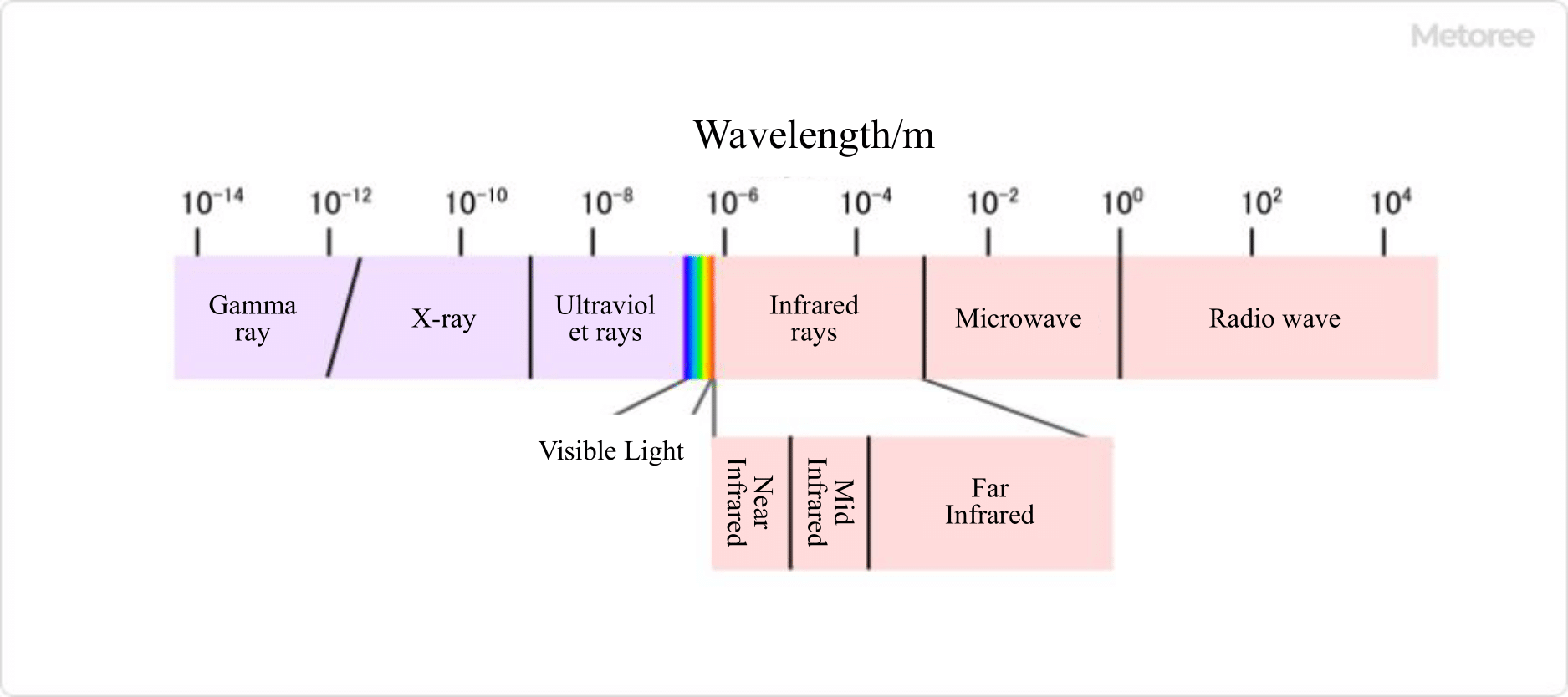
Figure2. Types of light
There are various types of Spectral Sensors, each of which can analyze different things. Here is a brief description of six typical types of instruments.
1. Ultraviolet-visible Spectrophotometer (UV-Vis)
This instrument uses ultraviolet or visible light as a light source to examine the light transmitted, absorbed, or reflected from a substance. It can be used for qualitative and quantitative analysis of components in a sample.
2. Infrared Spectrophotometer (IR)
This instrument uses infrared rays as a light source to examine the light transmitted through and reflected from a material. It can be used to estimate the structure and quantitatively analyze the components in a sample.
3. Inductively Coupled Plasma Atomic Emission Spectral Sensor(ICP-AES)
This is a device that detects the luminescence phenomenon generated when a sample is introduced into an inductively coupled plasma. It is extremely sensitive and can be used for qualitative and quantitative analysis of trace elements.
4. Atomic Absorption Spectral Sensor (AAS)
This device can perform qualitative and quantitative analysis of trace elements by utilizing the phenomenon that atoms absorb light at specific wavelengths.
5. X-ray Fluorescence Analyzer (XRF)
This device can perform elemental analysis of materials using X-rays as a light source. It can perform qualitative and quantitative analysis of samples by measuring the fluorescent X-rays specific to each element.
6. X-ray Photoelectron Spectral Sensor (XPS)
This device uses X-rays as a light source to obtain information on the atoms and molecules that make up the surface of a solid.
 Ein Tuner ist ein Gerät, das einen von mehreren über einen Fernseher oder ein Radio ausgestrahlten Kanälen auswählt.
Ein Tuner ist ein Gerät, das einen von mehreren über einen Fernseher oder ein Radio ausgestrahlten Kanälen auswählt. Ein Stichsägeblatt ist das Blatt einer elektrischen Säge, die als Stichsäge bezeichnet wird. Je nachdem, ob es sich bei dem zu schneidenden Material um Holz oder Metall handelt, wird das entsprechende Blatt verwendet. Durch den Austausch des Sägeblatts kann die Palette der zu bearbeitenden Materialien und der zu bearbeitenden Formen erweitert werden. Stichsägeblätter können auch ersetzt werden, wenn sie abgenutzt sind.
Ein Stichsägeblatt ist das Blatt einer elektrischen Säge, die als Stichsäge bezeichnet wird. Je nachdem, ob es sich bei dem zu schneidenden Material um Holz oder Metall handelt, wird das entsprechende Blatt verwendet. Durch den Austausch des Sägeblatts kann die Palette der zu bearbeitenden Materialien und der zu bearbeitenden Formen erweitert werden. Stichsägeblätter können auch ersetzt werden, wenn sie abgenutzt sind.


