What Is Sulfur Trioxide?
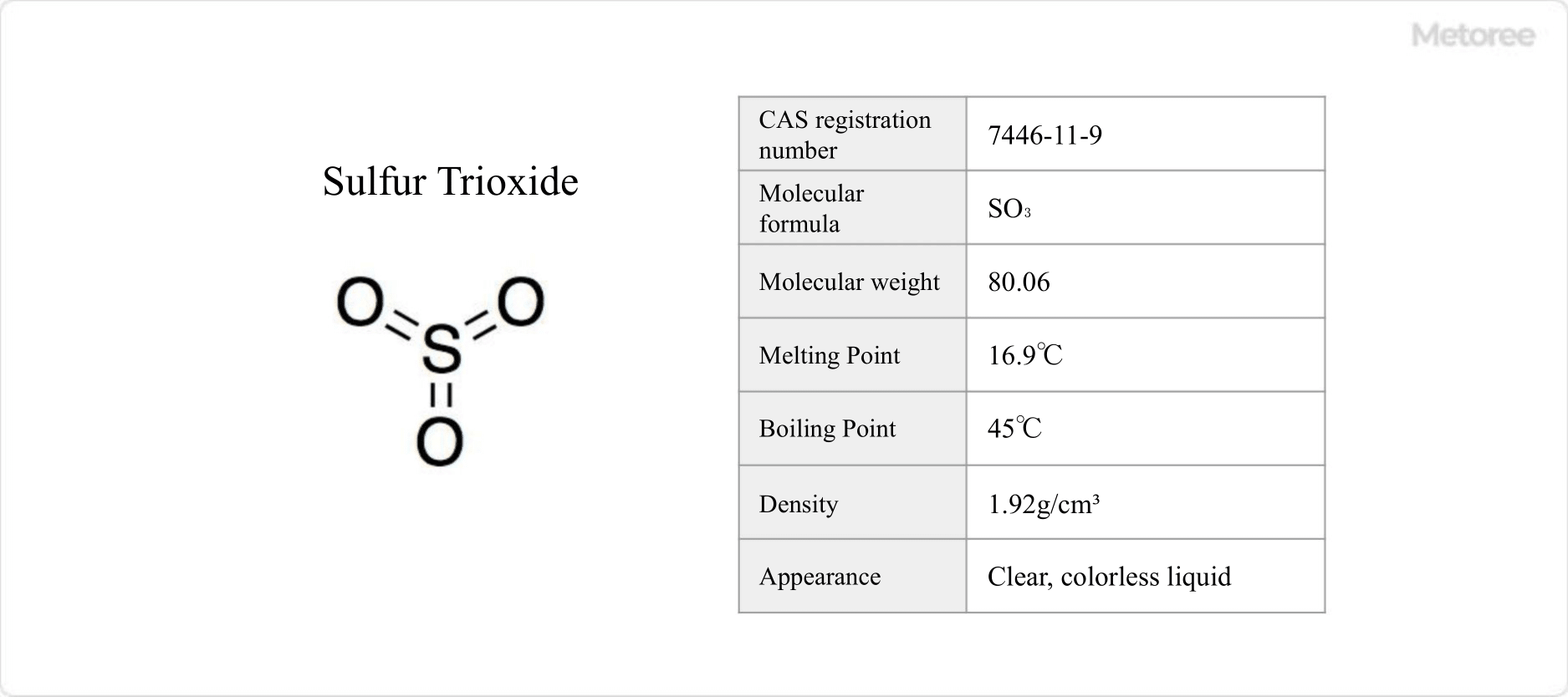
Figure 1. Basic Information on Sulfur Trioxide
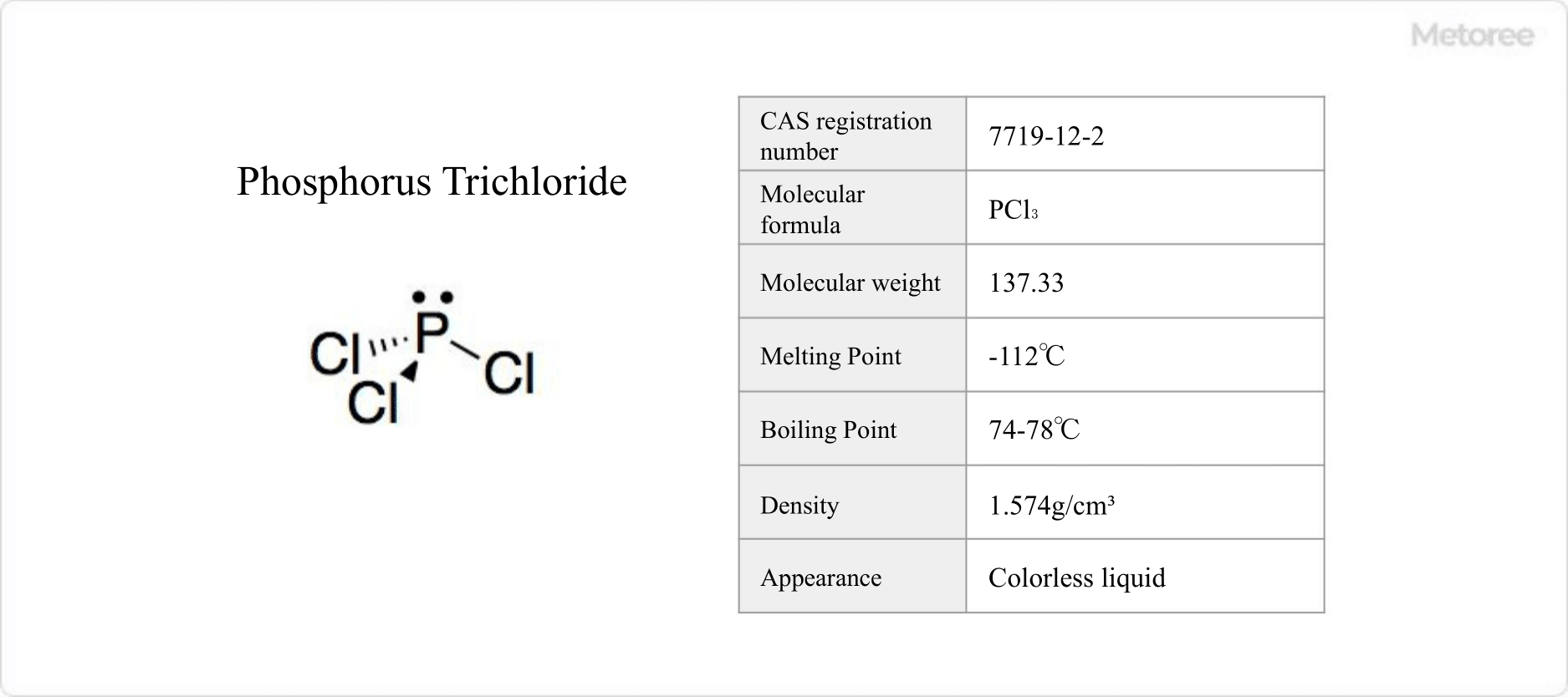
Sulfur trioxide is an oxide of sulfur with the chemical formula SO3.
It is synonymous with anhydrous sulfuric acid and serves as an intermediate in the contact process for manufacturing sulfuric acid.
Classified by the GHS as hazardous, sulfur trioxide poses risks such as severe skin and eye irritation, respiratory tract irritation, and potential dental damage from prolonged or repeated exposure. It is a specified substance under the Air Pollution Control Law but is exempt from several other regulations including the Industrial Safety and Health Law and the Poisonous and Deleterious Substances Control Law.
Uses of Sulfur Trioxide
Sulfur trioxide acts as an oxidizing and sulfonating agent, used in manufacturing ion exchange resins and sulfuric acid. Its role as a sulfonating agent extends to the industrial synthesis of dyes and neutral detergents.
As an intermediate in the contact process, sulfur trioxide is formed from sulfur dioxide in the presence of a catalyst and is crucial for producing high-purity concentrated sulfuric acid.
Properties of Sulfur Trioxide
Sulfur trioxide, a clear, colorless liquid at room temperature, has a melting point of 16.9°C and a boiling point of 45°C. It emits a strong pungent odor and forms fuming sulfuric acid when dissolved in concentrated sulfuric acid.
Its high hygroscopic nature means that sulfur trioxide can cause wood and cotton to ignite by dehydrating the water content, thus increasing flammability.
Structure of Sulfur Trioxide
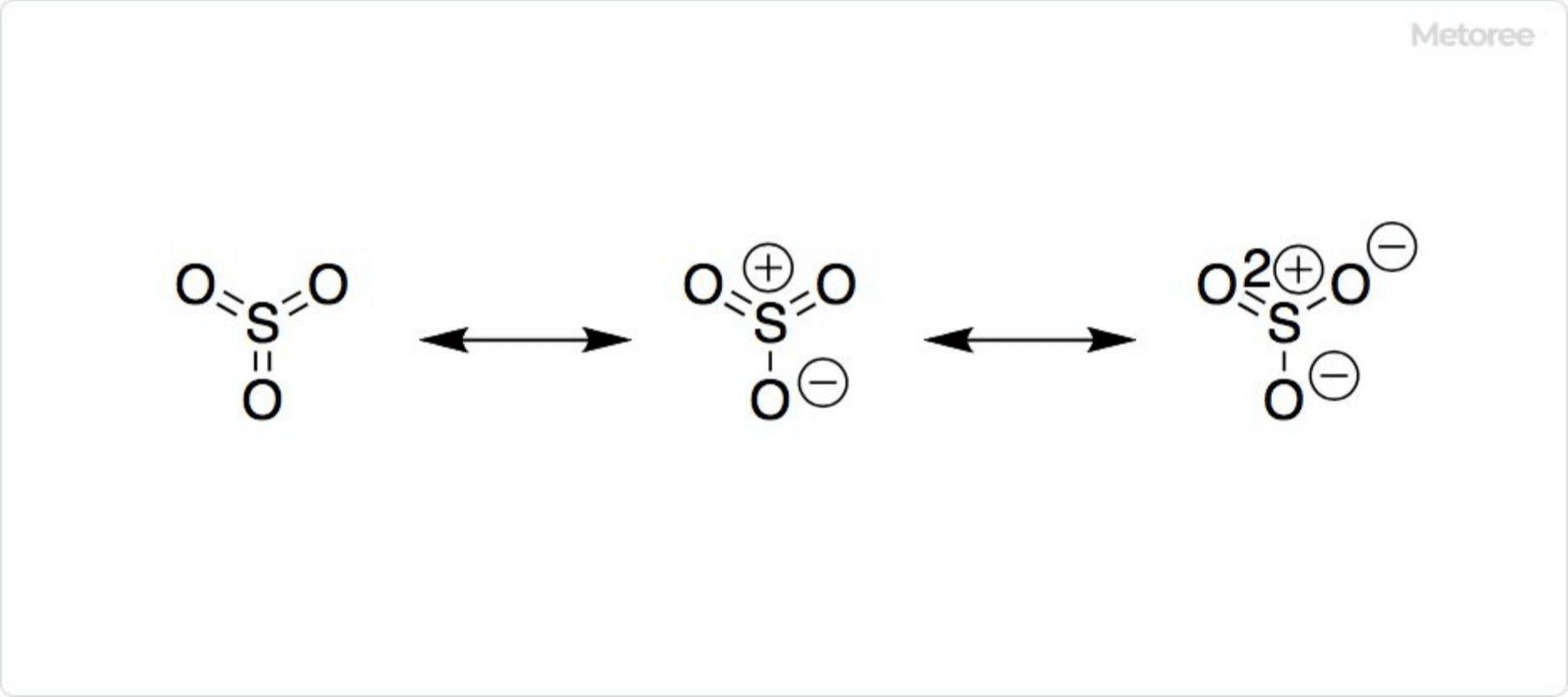
Figure 2. Structure of Sulfur Trioxide
With a molar mass of 80.06 g/mol and a density of 1.92 g/cm3, sulfur trioxide typically has a planar equilateral triangle structure. The sulfur atom, at the center, exhibits an oxidation number of +6 and is surrounded by six electron pairs, most of which are non-bonding in nature. The three S-O bonds are equal in length, at 1.42 Å, and form a 120° angle (∠O-S-O).
Types of Sulfur Trioxide
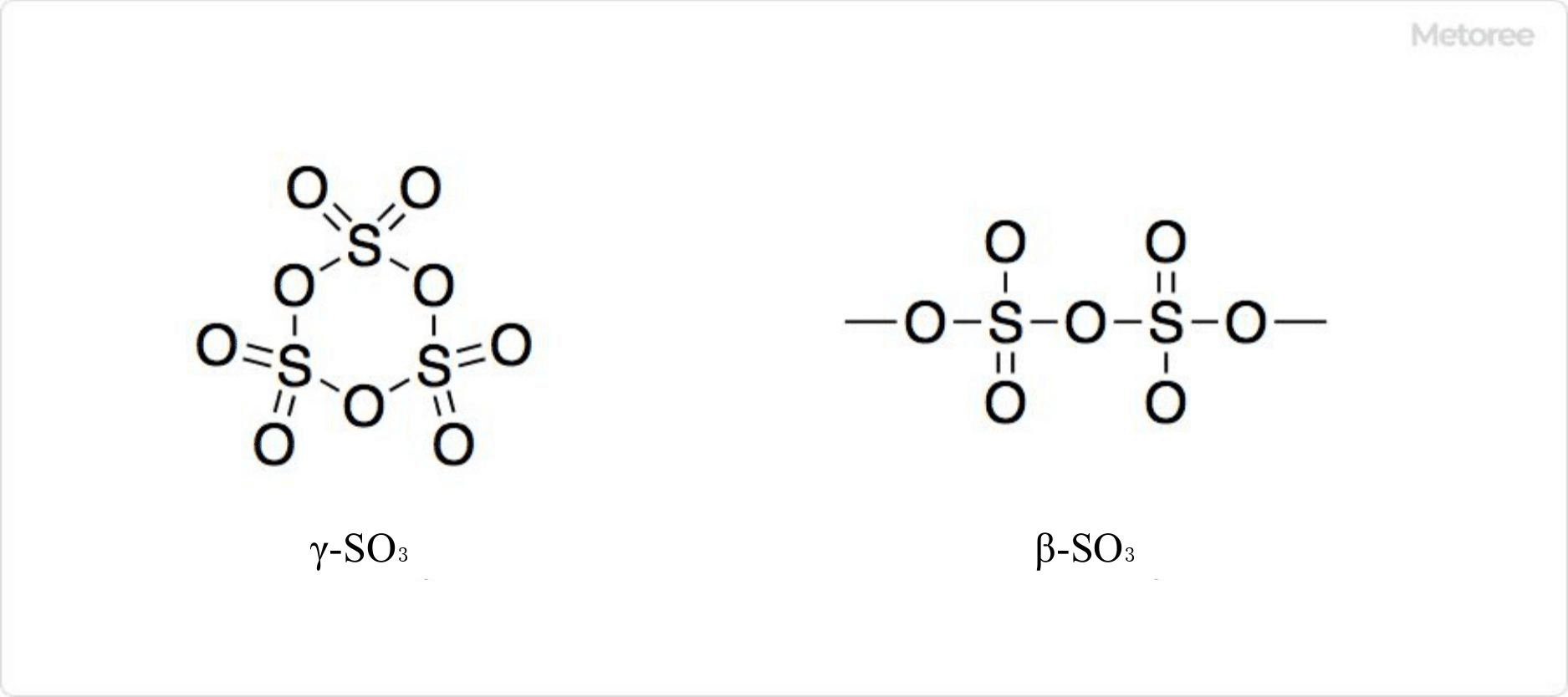
Figure 3. Types of Sulfur Trioxide
Sulfur trioxide exists in three polymorphic forms: α-SO3, β-SO3, and γ-SO3, each exhibiting unique behavior in the presence of trace amounts of water. The γ form aggregates to form a molecular crystal, while α and β forms can transition to each other under specific conditions, showcasing a range of structural complexities from fibrous to helical arrangements.
Other Information on Sulfur Trioxide
1. Synthesis of Sulfur Trioxide
Laboratory synthesis of sulfur trioxide involves the thermal decomposition of sodium hydrogen sulfate, among other methods. Industrially, the contact method predominates, involving the oxidation of sulfur dioxide at high temperatures to produce sulfur trioxide.
The lead chamber process, once a common method for sulfuric acid production, has become obsolete with the advent of the more efficient contact method.
2. Reaction of Sulfur Trioxide
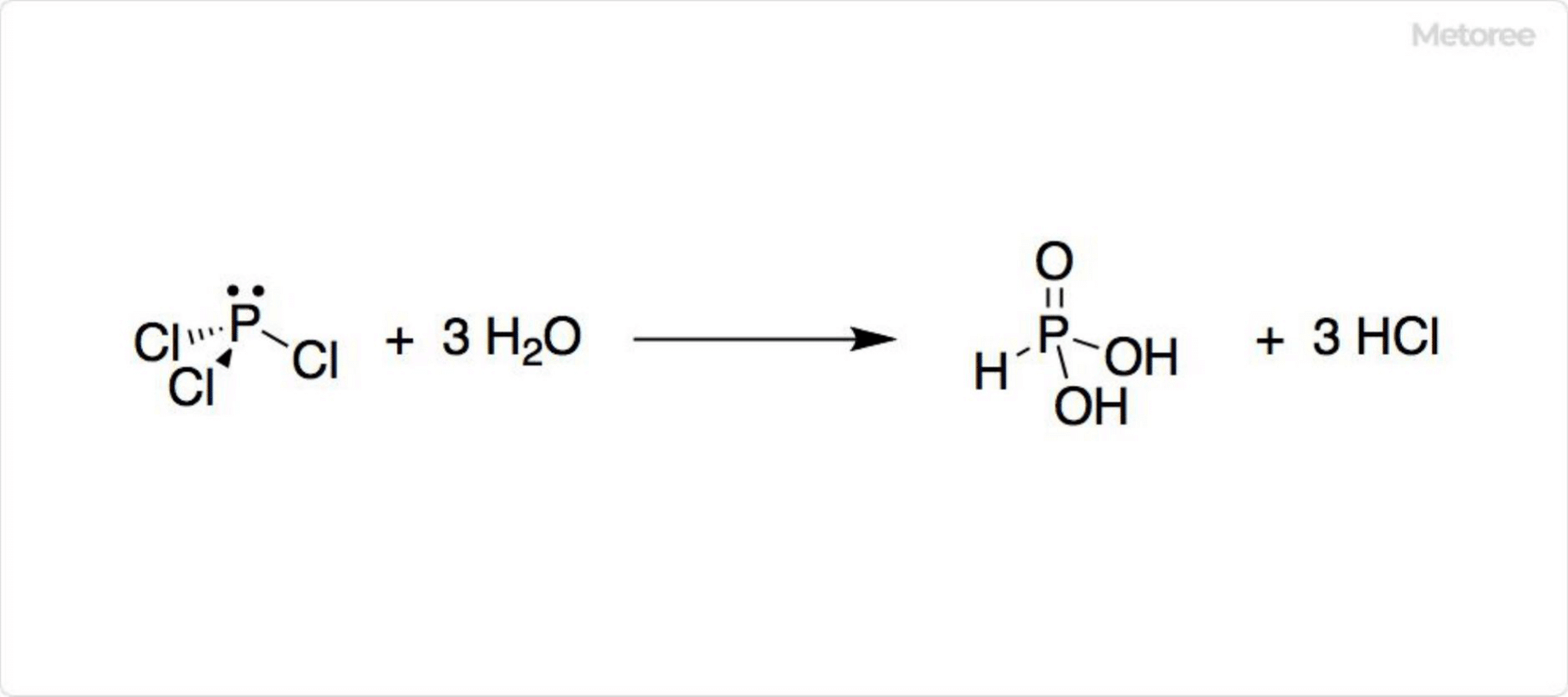
As an anhydride of sulfuric acid, sulfur trioxide reacts exothermically with water, contributing to acid rain. It is also involved in the production of thionyl chloride through the reaction with sulfur dichloride.