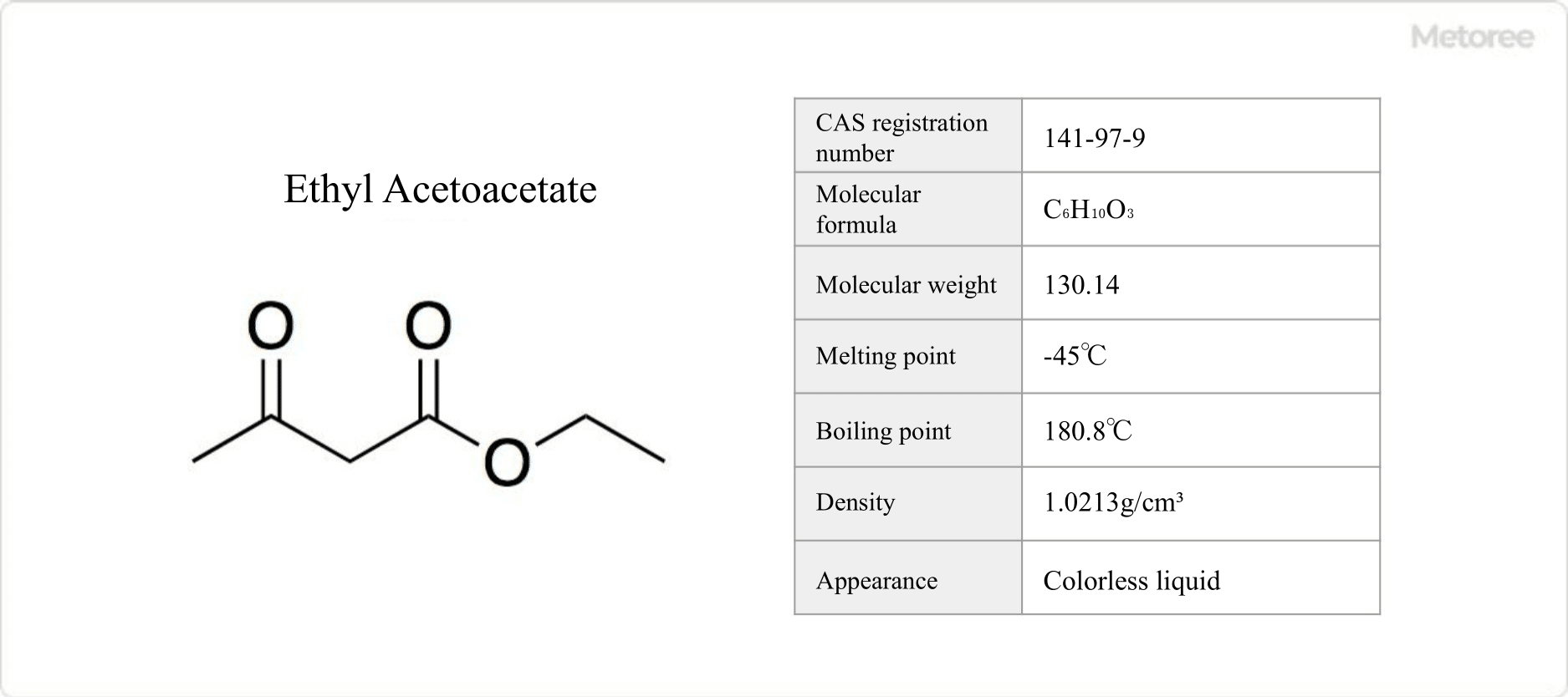
What Is Antimony?
Antimony is a rare metal with a silvery-white luster.
Antimony has the element symbol Sb, atomic number 51, and CAS number 7440-36-0. It can be obtained from naturally occurring ores, and China is the main producer in the world.
Properties of Antimony
1. Physical Properties
Antimony has a melting point of 630°C, a boiling point of 1,635°C, and a relative density of 6.7. Like most metals, it is virtually insoluble in water and organic solvents but can be dissolved in royal water.
2. Other Characteristics
Antimony has the following characteristics
- It is brittle and can be pounded to powder.
- Its volume increases when it solidifies.
- Hardness increases when alloyed with copper, tin, lead, etc.
- Toxic and bactericidal.
Taking advantage of these characteristics, antimony is used in various fields.
Uses of Antimony
Antimony is used mainly in industrial products as a material for semiconductors, electrodes, and alloys, as well as in automobiles, office automation equipment, home appliances, and many other products.
1. Flame Retardants
Antimony is mainly used as a flame retardant in combination with halogen-based flame retardants, except for halogen-containing polymers, where antimony trioxide (SbO3), a flame retardant compound, is used. The flame retardant effect of antimony trioxide is due to the formation of halogenated antimony compounds that react with hydrogen and oxygen atoms and OH radicals to suppress fires. Antimony compounds are used in applications to create flame-retardant materials, such as in children’s clothing, toys, aircraft, and automobile seat covers, as well as in polyester resins added to fiberglass composite materials such as engine covers for light aircraft.
2. Alloying Materials
Antimony can form very useful alloys with lead, increasing its hardness and mechanical strength, so various amounts of antimony are used as an alloying metal in most applications involving lead. For example, it can be added to the electrodes of lead-acid batteries to improve plate strength and charging properties and is also used in batteries to improve battery performance. Antimony is used in antifriction alloys (such as Babbitt metals), bullets, electrical cable coatings, type alloys, solders, pewter, and low-hardness hardening alloys.
3. Other
Antimony is used as an additive in engine block casting, antifriction material in brakes, wiring cords, and rubber parts, to name a few automotive applications. Other applications include stabilizers and catalysts in polymer production, semiconductor materials, and emetics.
Other Information on Antimony
1. Antimony Manufacturing Process
The extraction of antimony from ores depends on the quality and composition of the ore, but most antimony is mined as sulfide (stibnite). Antimony can be isolated from crude antimony sulfide by reduction with iron (Sb2S3+3Fe→2Sb+3Fe). It can also be isolated from oxides by carbothermic reduction (2Sb2O3+3C→4Sb+3CO2).
2. Regulatory Information
Antimony is not designated under the Poisonous and Deleterious Substances Control Law, but its powdered form is classified as a “Hazardous Substance, Class II, Metallic Powder” under the Fire Service Law. Antimony is designated as a “Class 1 Designated Chemical Substance (Article 2, Paragraph 2 of the Law)” under the Act on Confirmation, etc. of Release of Specific Chemical Substances and Promotion of Chemical Management (PRTR Law) and as a “Hazardous and Noxious Substance to be Labeled or Notified” under the Industrial Safety and Health Law.
3. Handling and Storage Precautions
Precautions for handling and storage are as follows
- Keep the container tightly closed and store it in a dry, cool, and dark place.
- Use only outdoors or in well-ventilated areas.
- Avoid contact with hot surfaces, sparks, and naked flames as there is a risk of ignition.
- Avoid mixing with oxidizers such as halogen, alkali permanganate, or metal powders due to the risk of fire or explosion.
- Avoid contact with acids due to the risk of toxic gas generation.
- Wear protective gloves and glasses when using.
- Wash hands thoroughly after handling.
- In case of skin contact, rinse immediately with water.
- In case of eye contact, rinse cautiously with water for several minutes.