What Is Cyclohexane?
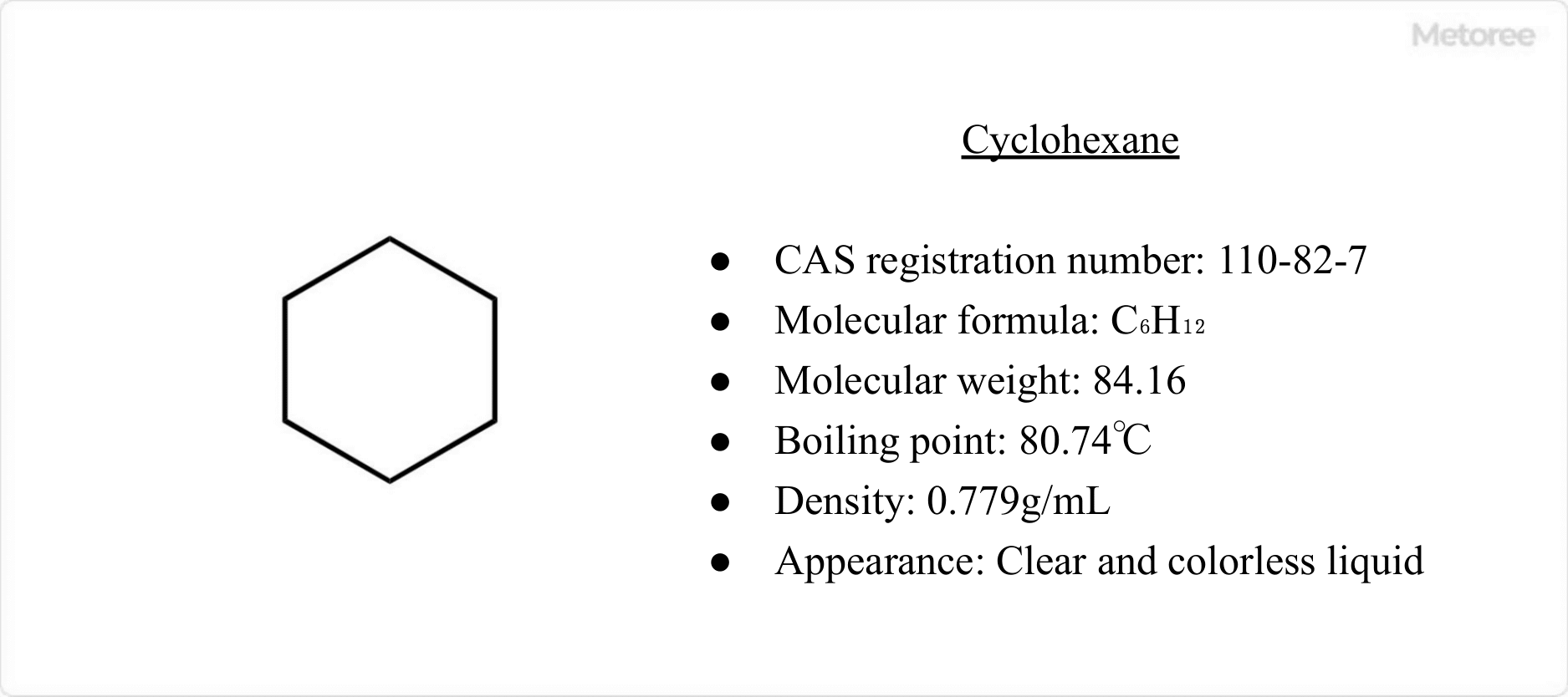
Figure 1. Basic information on cyclohexane
Cyclohexane is a type of cycloalkane formed by hydrogenation of benzene, etc. It is widely used as a solvent. It is also called hexamethylene or hexahydrobenzene.
Cyclohexane is a colorless liquid with an odor similar to gasoline. It has a molecular formula of C6H12, a molecular weight of 84.16, a boiling point of 80.74°C, and a density of 0.779 g/mL.
Cyclohexane exists in stereoisomers called chair, boat, half chair, and twisted boat forms, with the chair form being the most stable.
Uses of Cyclohexane
Cyclohexane is used as a raw material for caprolactam and adipic acid, which are synthetic intermediates of nylon, and is most commonly used in the manufacture of nylon.
Other uses include solvents for paints, ethers, waxes, and rubbers, oil and fat extraction, paint and varnish removers, solvent adhesives, and aerosol adhesives. As a solvent, it can be widely used for synthesis, cleaning, and other applications, as well as for analytical purposes, such as eluents for liquid chromatography analysis.
Properties of Cyclohexane
Cyclohexane is insoluble in polar solvents and soluble in organic solvents. It is highly volatile and extremely flammable.
Because of its anesthetic properties, it must be handled with care. For example, prolonged contact of cyclohexane with the skin, for example, can cause illnesses such as dermatitis. When inhaled, low concentrations can cause headaches, and high concentrations can cause disorientation. Particular attention should be paid to low concentrations, as it has almost no odor.
Structure of Cyclohexane
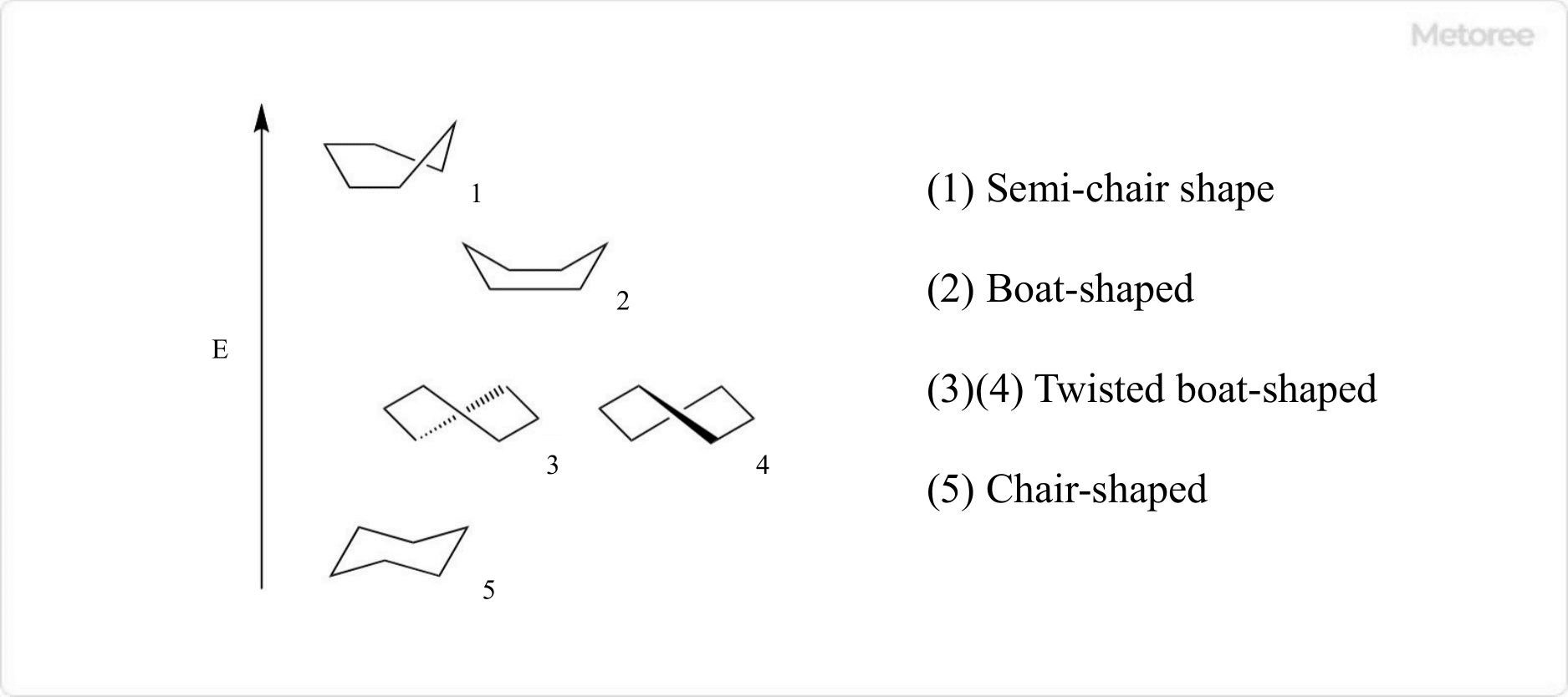
Figure 2. Conformation of cyclohexane
The most stable conformation of cyclohexane is chair-shaped, followed by twisted boat-shaped, boat-shaped, and semi-chair-shaped.
In the case of cyclohexane rings with substituents, other conformations may be more stable due to steric hindrance caused by the bulkiness of the substituents.
Other Information on Cyclohexane
1. Synthesis of Cyclohexane
Industrially, the majority of cyclohexane is produced by catalytic hydrogenation of benzene using palladium or nickel catalysts. Methylcyclopentane, produced in the process of petroleum reforming, can also be converted to cyclohexane using a catalyst.
Industrially obtained cyclohexane is converted to cyclohexanol and cyclohexanone, which are eventually converted to ε-caprolactam, hexamethylenediamine, and adipic acid. These compounds can be used as raw materials for 6-nylon and 6,6-nylon.
2. Ring inversion of Cyclohexane
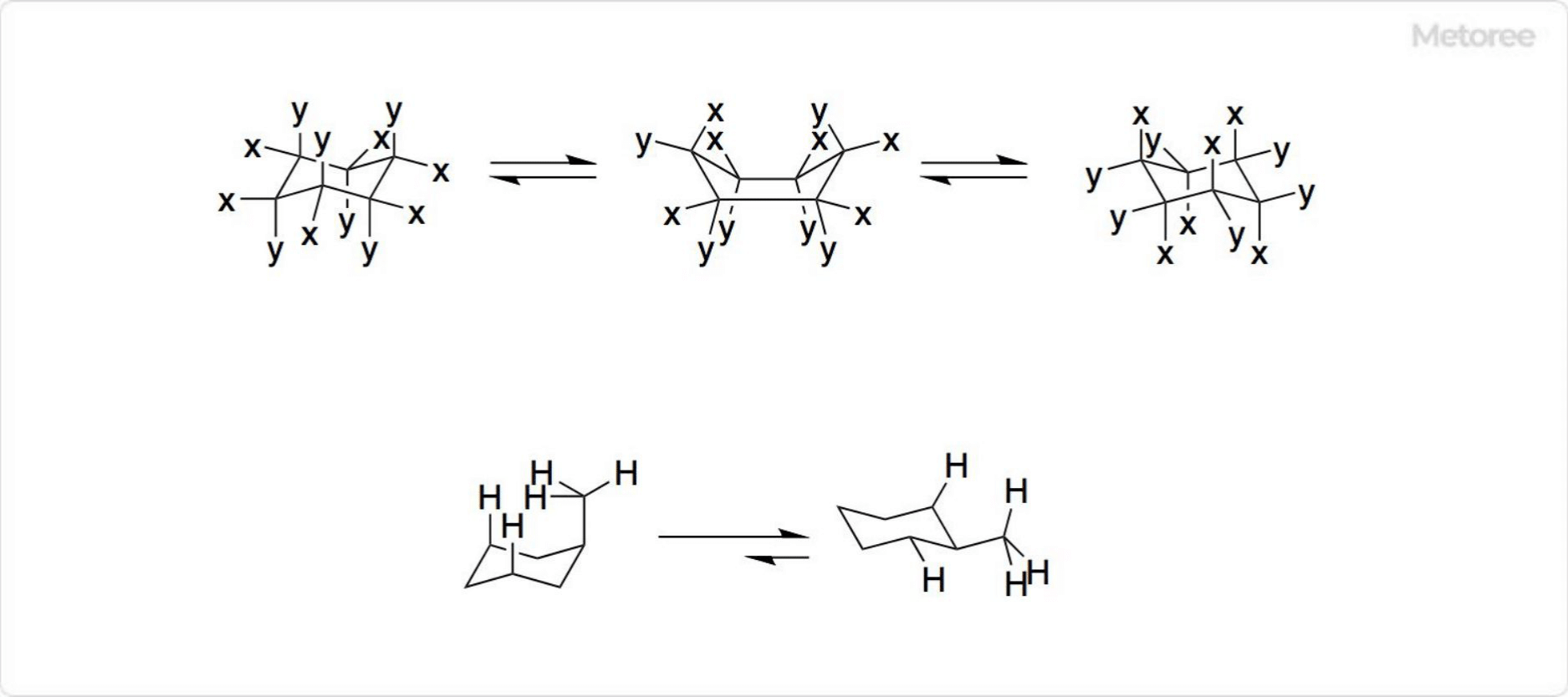
Figure 3. Ring inversion of cyclohexane
In the chair conformation of cyclohexane and other cyclic compounds with a cyclohexane-type structure, a distinction is made between substituents parallel and perpendicular to the ring plane. Substituents parallel to the ring plane are called equatorial, and those perpendicular to the ring plane are called axial.
Because of free rotation at each of the bonding axes that form the ring, cyclohexane goes through a fuselage conformation and the two chair-shaped conformations swap places with each other. This is called ring inversion. Through ring inversion, it is possible for an equatorial to change to an axial orientation and an axial to an equatorial orientation.
3. Cyclohexane Ring With Bulky Substituents
The distance between substituents is closer in the axial form than in the equatorial form. In the case of cyclohexane rings with bulky substituents, it is also known that the substituents avoid the axial form and predominantly take the equatorial form, since this affects the stability of the stereo conformation.