What Is Polyurethane?

Polyurethane is a polymer with urethane bonds obtained by reacting isocyanate groups with hydroxyl groups.
By changing the type and composition of the primary raw materials and the molding method, polyurethane can be made spongy, flexible like rubber, or hard like rubber tires.
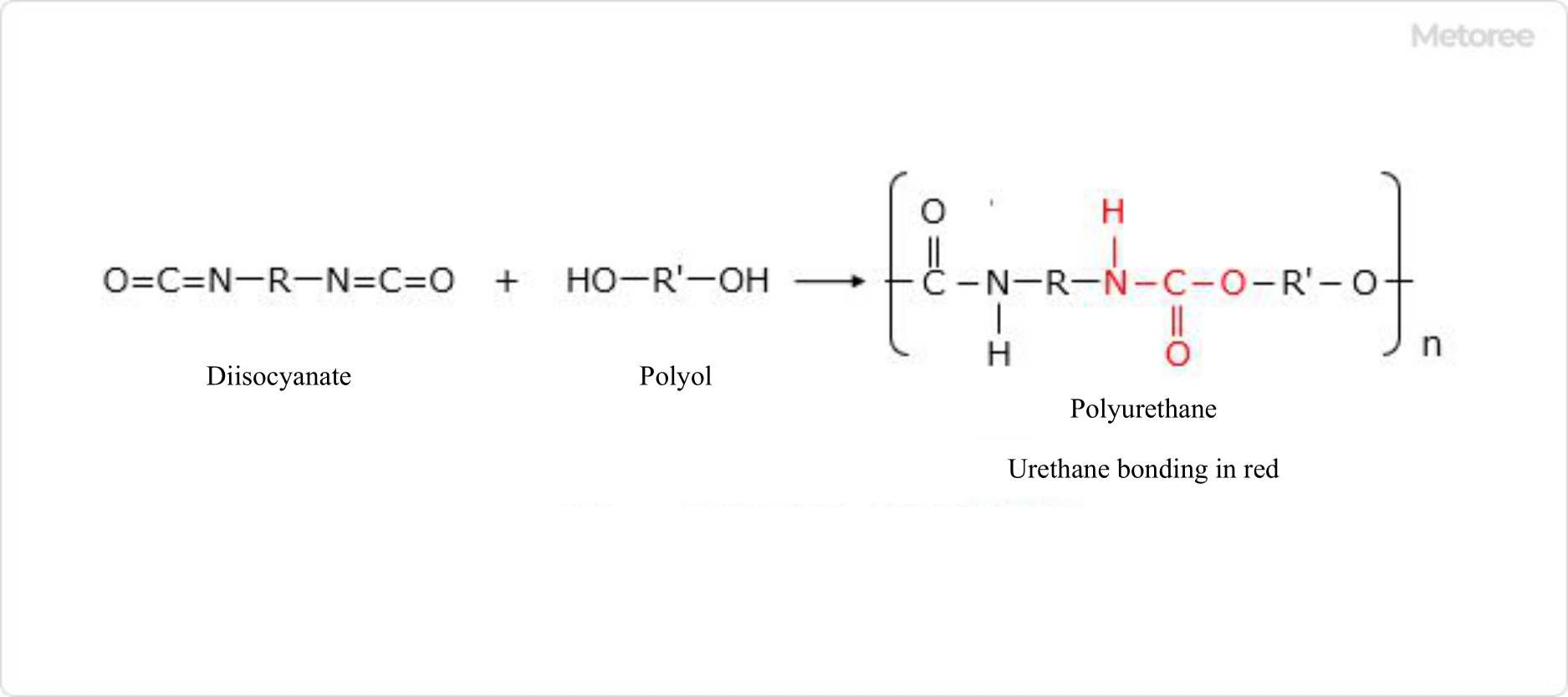
Figure 1. Polyurethane synthesis reaction formula
Uses for Polyurethane
Polyurethane can be divided into two broad categories in terms of appearance: foam-based, which is porous, and non-foam-based, which is rubber-like. Since the uses for each are quite different, they are described separately below.
1. Foam-Based
Foam-based polyurethane comes in two types: soft foam and hard foam. Soft foam, in particular, is used in various applications, from everyday items to industrial uses. Soft foam applications include sponges used in kitchens, cushions for headphones and other devices, rollers for industrial equipment, and soundproofing materials.
Rigid polyurethane foam applications are often used in construction sites as heat insulators.
2. Non-foam Type
Non-foamed polyurethane has high elasticity and toughness and is used as elastic structural materials such as rubber and elastomers, as well as fibers, paints, and adhesives.
As elastomers, they are used in tires, belts, gaskets, rollers, machine parts, and automobile bumpers. Polyurethane fibers, called spandex, are also widely used in clothing such as jackets, pants, swimwear, sportswear, etc., due to their high elasticity.
Composition of Polyurethane
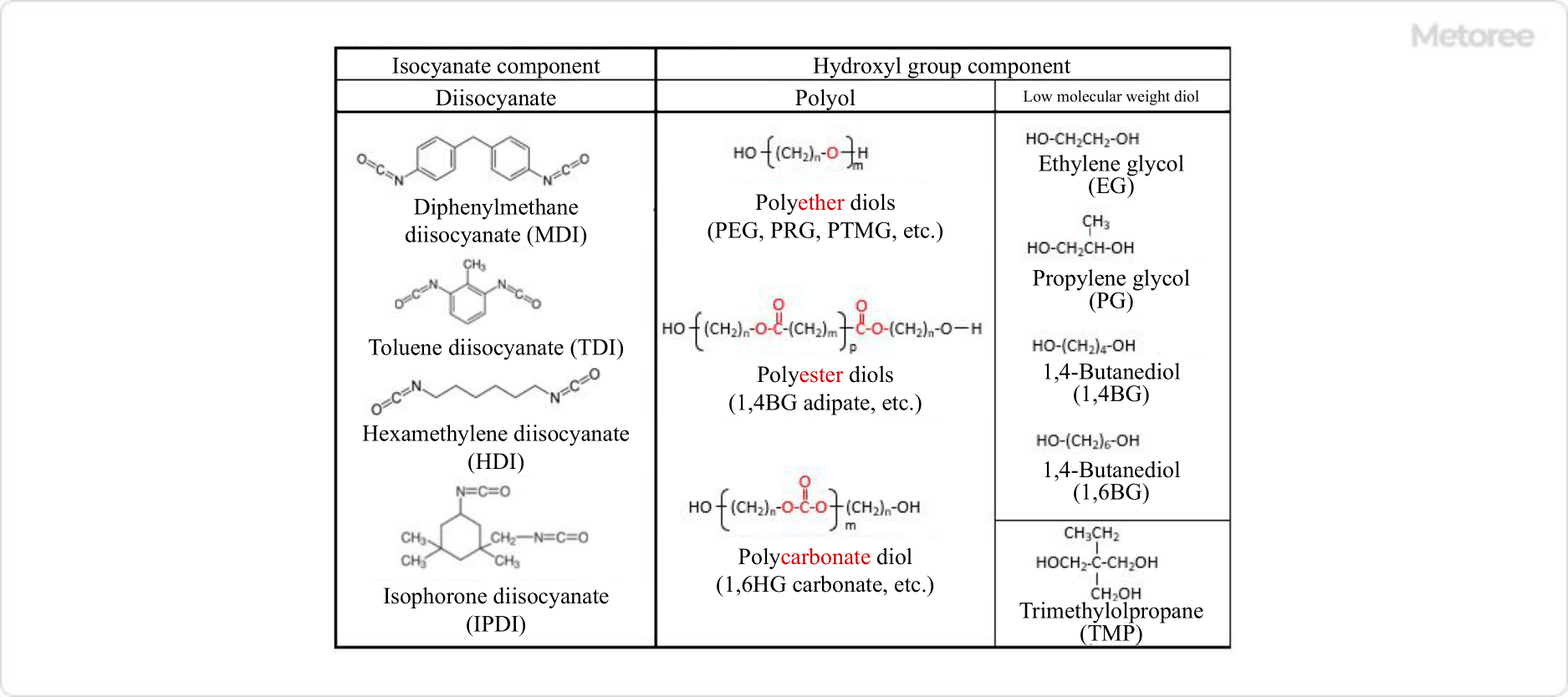
Figure 2. Typical examples of raw materials that make up polyurethane
The raw materials used to make polyurethane include diisocyanates, which have bifunctional isocyanate groups in their molecules, polyols, which have hydroxyl groups in their molecules, and low molecular weight diols.
While there are only a few types of diisocyanates used in polyurethane, there is a very wide range of polyols, which are the raw materials for the hydroxyl group component. This is because polyols themselves are polymers with molecular weights ranging from several hundred to several thousand, and they are composed of a wide range of monomer combinations.
The low molecular weight diols are typical examples of hydroxyl group components, and their dimers are also used as low molecular weight diols. Triols with three hydroxyl groups and alcohols with a single hydroxyl group are also used to adjust the molecular weight.
Characteristics of Polyurethane
The characteristics of polyurethane vary greatly depending on the type of raw material used in the mixture. In general, polyurethanes have excellent mechanical strength (elasticity and toughness) and the following characteristics:
1. Advantages
- High mechanical strength, excellent elasticity and toughness, and high tensile strength. Maintains elasticity even at high hardness.
- Excellent abrasion resistance and aging resistance.
- Excellent oil and solvent resistance, and good adhesion.
- Excellent low-temperature properties and good weather resistance.
- Highly resilient to compression.
2. Disadvantages
- Poor heat resistance; continuous use temperature is limited to 80°C to 100°C.
- Easily hydrolyzed and weak in water.
- Toxic gases are generated by combustion.
These characteristics vary greatly depending on the combination of raw materials used. For example, hardness and strength differ greatly depending on the ratio of polyol and low molecular weight diol used. In addition, the polyol component contributes significantly to weather resistance and hydrolysis resistance. Therefore, it is important to design molecules that match the required performance.
Other Information on Polyurethane
Production Methods for Polyurethane Foam
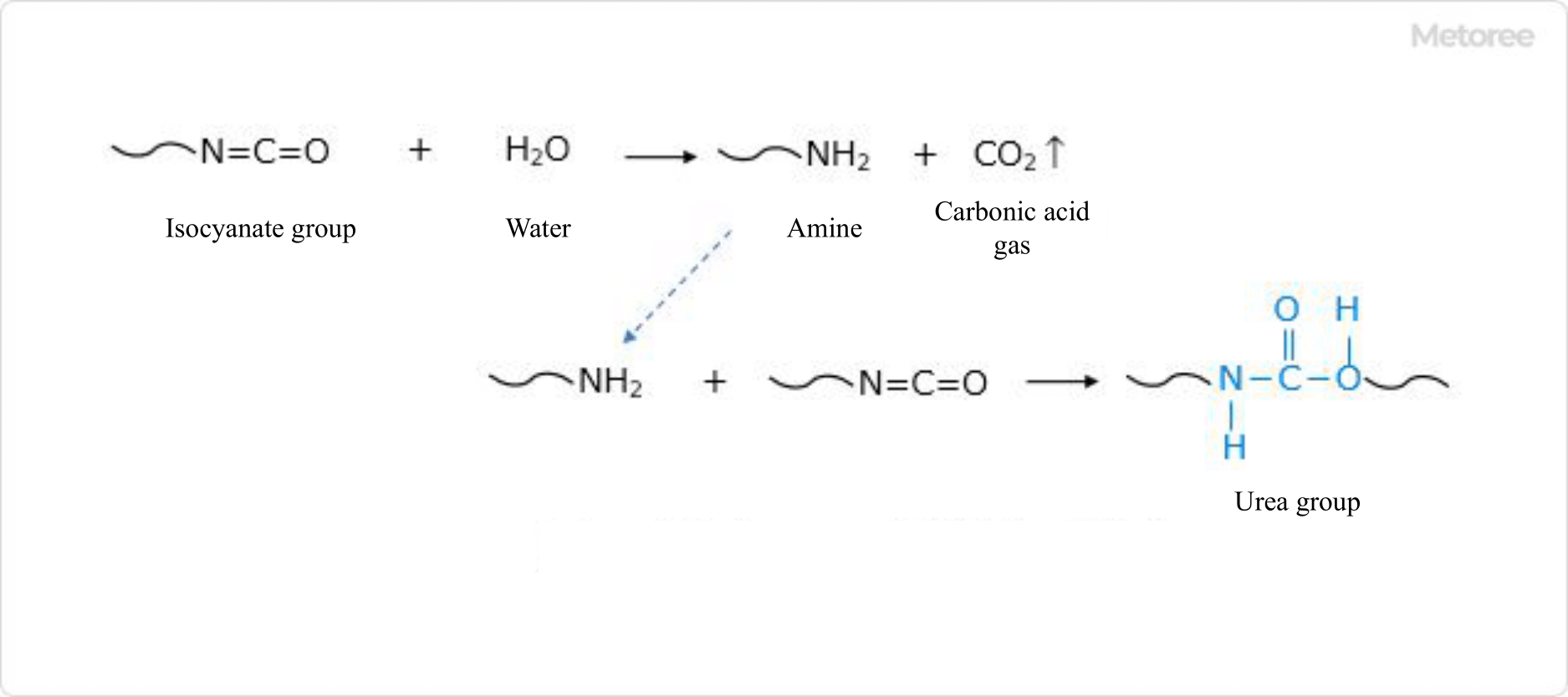
Figure 3. Reaction Formula for Urethane Foam Foaming
Polyurethane foam is a sponge-like molded product produced by foaming polyurethane during the molding process. There are multiple methods of manufacturing polyurethane foam, including slab molding, in which a mixture of raw materials is foamed without being placed in a mold and then cut into the shape of the product. Other methods are mold molding, where a mold is used to form the product into the required shape, and laminate molding, which is useful for producing large insulation boards.
1. Soft Foam-Based Polyurethane
In soft foam polyurethane, water is added as a foaming agent to the raw material polyol, and the isocyanate groups react with the water to produce carbon dioxide gas. At the same time, resinification by the reaction of polyol, low molecular weight diol, and isocyanate proceeds, so that each bubble of carbon dioxide gas cures in a continuous state to form a porous foam.
During the reaction with water, the isocyanate becomes an amine. However, due to the extremely high reactivity of amines, it immediately reacts with another isocyanate group to form a urea bond. For this reason, the composition of soft foam-based polyurethane contains not only urethane bonds but also many urea bonds.
2. Rigid-Foam Polyurethanes
Rigid-foam polyurethane uses a low-boiling physical foaming agent that evaporates from the reaction heat during urethane conversion. The bubbles of rigid foam are independent bubbles to obtain a high thermal insulation effect.