What Is Ferric Chloride Etching?
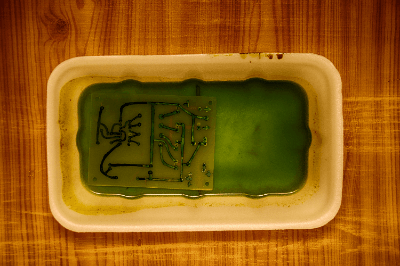
Ferric chloride etching is a method of wet etching, a process used to shape the surface of materials such as metals, glass, and ceramics. This technique involves removing material through erosion by solutions, differing from dry etching, which uses gases and beams.
Ferric chloride (FeCl3) solution is used in this wet etching method, being suitable for almost all metals except a few, like titanium.
Uses of Ferric Chloride Etching
Ferric chloride etching is widely used in metal processing for its precision compared to traditional machining methods. It’s particularly prevalent in the electronics industry for creating detailed wiring patterns on printed circuit boards (PCBs) and manufacturing IC lead frames. This technique is also used in the fine processing of metals and ceramics in various manufacturing industries and art for copper plate etching in printmaking.
Principle of Ferric Chloride Etching
The process begins by applying a resist to parts of the metal surface that should remain unetched. On PCBs, this is typically done using metal resists like solder or tin, or organic resists such as ink or film.
When the material is immersed in ferric chloride solution, an oxidation-reduction reaction occurs, where copper transforms into cuprous chloride (CuCl), and ferric chloride reduces to ferrous chloride (FeCl2). This reaction involves electron exchange between copper atoms and ferric ions, resulting in the removal of copper from the surface.
After etching, the resist is stripped using acidic solutions for metal resists or alkaline solutions for organic resists. Finally, the substrate is cleaned to remove any remaining chemicals or resist residues.