What Is Chloroacetic Acid?
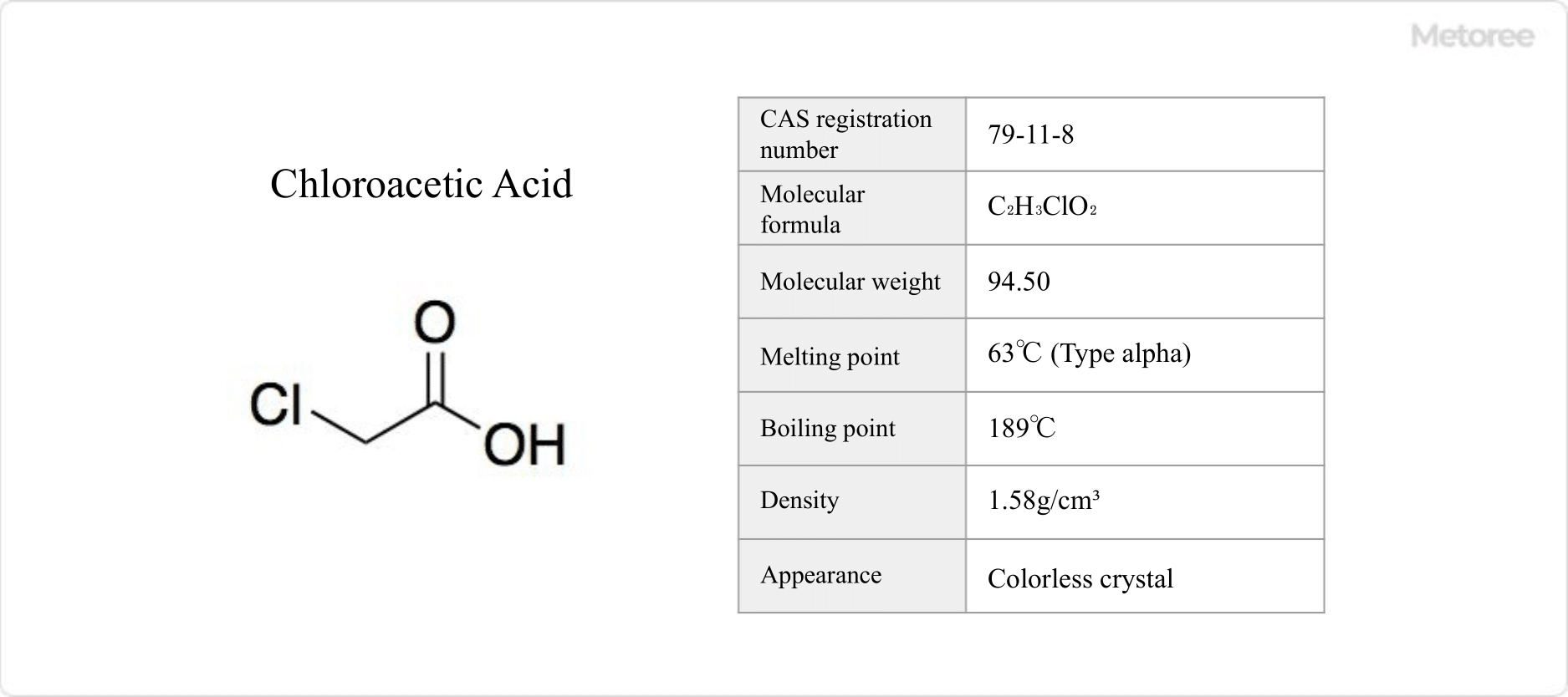
Figure 1. Basic information on chloroacetic acid
Chloroacetic acid is an organic compound with the chemical formula C2H3ClO2.
When the number of substituted hydrogen atoms is emphasized, it is called monochloroacetic acid.
Uses of Chloroacetic Acid
Chloroacetic acid is used in herbicides and surfactants. Because of its strong acidity, it is used as a remedy to remove warts. When applied to warts, it removes them by corroding and necrotic tissue, but it should be handled with care because it can cause blisters and wounds when it adheres to skin and mucous membranes.
In tap water, chlorine, a disinfectant, reacts with organic matter in tap water to produce chloroacetic acid as one of the disinfection byproducts. Chloroacetic acid is also used in the synthesis of amino acids and carboxymethyl cellulose.
Properties of Chloroacetic Acid
Chloroacetic acid has a pungent odor similar to that of acetic acid and is available in three forms, with melting points of 63°C for the alpha form, 55-56°C for the beta form, and 50°C for the gamma form. It is a clear colorless solid with tidal solubility and a boiling point of 189°C. It can be used in water, ethanol, chloroform, and chloroform.
It is soluble in water, ethanol, chloroform, benzene, and ether. In aqueous solution, it is a stronger acid than acetic acid, pKa = 2.85 at 25°C.
Structure of Chloroacetic Acid
Chloroacetic acid has a molecular weight of 94.50 and a density of 1.58.
The differential formula is expressed as CH2ClCOOH.
Other Information on Chloroacetic Acid
1. History of Chloroacetic Acid
Chloroacetic acid with impurities was first prepared by Félix LeBlanc in 1843 by chlorinating acetic acid in the presence of sunlight.
In 1857, Reinhold Hoffmann refluxed chlorine and glacial acetic acid in the presence of sunlight to obtain pure chloroacetic acid. In the same year, Charles Adolphe Wurtz also synthesized chloroacetic acid by hydrolysis of chloroacetyl chloride (ClCH2COCl).
2. Synthesis of Chloroacetic Acid
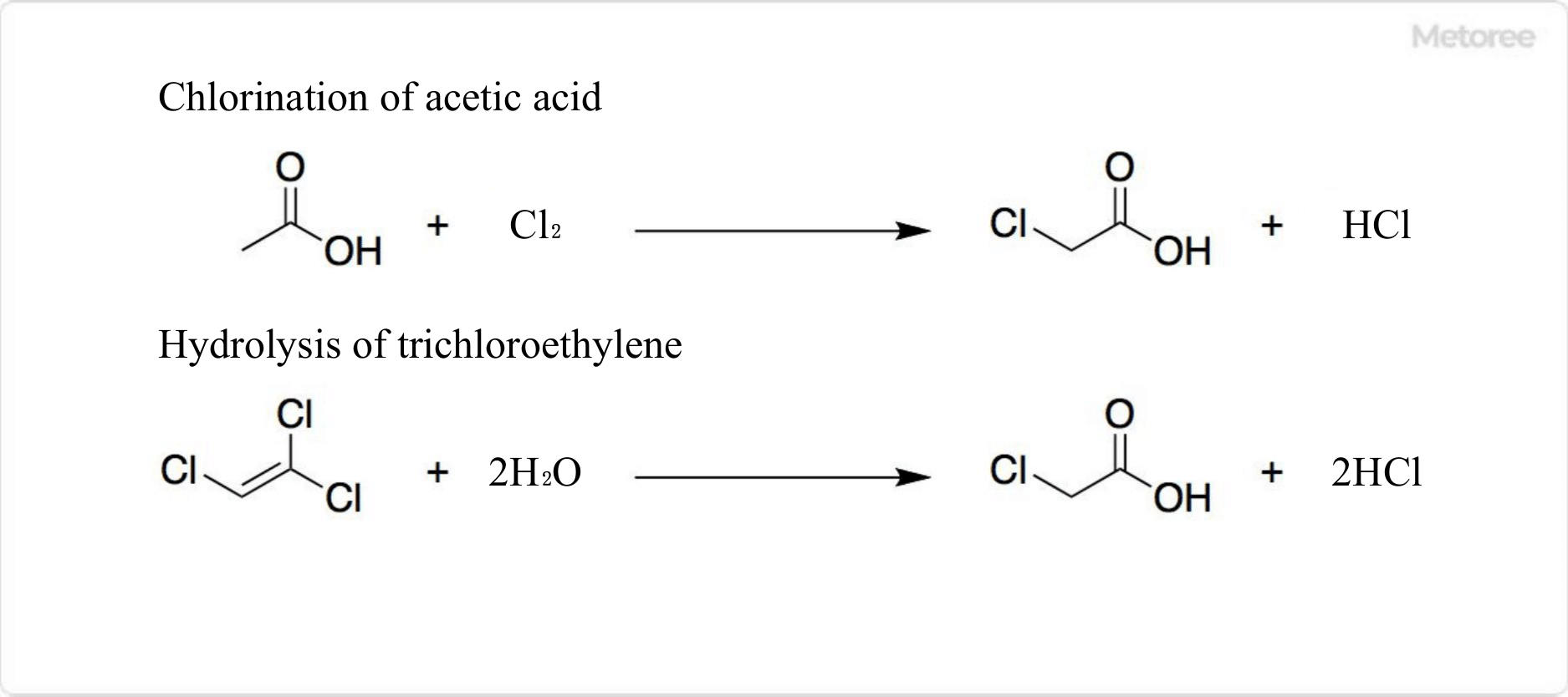
Figure 2. Synthesis of chloroacetic acid
Approximately 420,000 tons of chloroacetic acid are produced annually worldwide. Chloroacetic acid is obtained by chlorination of acetic acid using acetic anhydride as a catalyst.
Chloroacetic acid can also be produced by hydrolyzing trichloroethylene in concentrated sulfuric acid at 130-140°C. This reaction yields a more pure Chloroacetic Acid than halogenation, but a large amount of HCl is released.
3. Reaction of Chloroacetic Acid
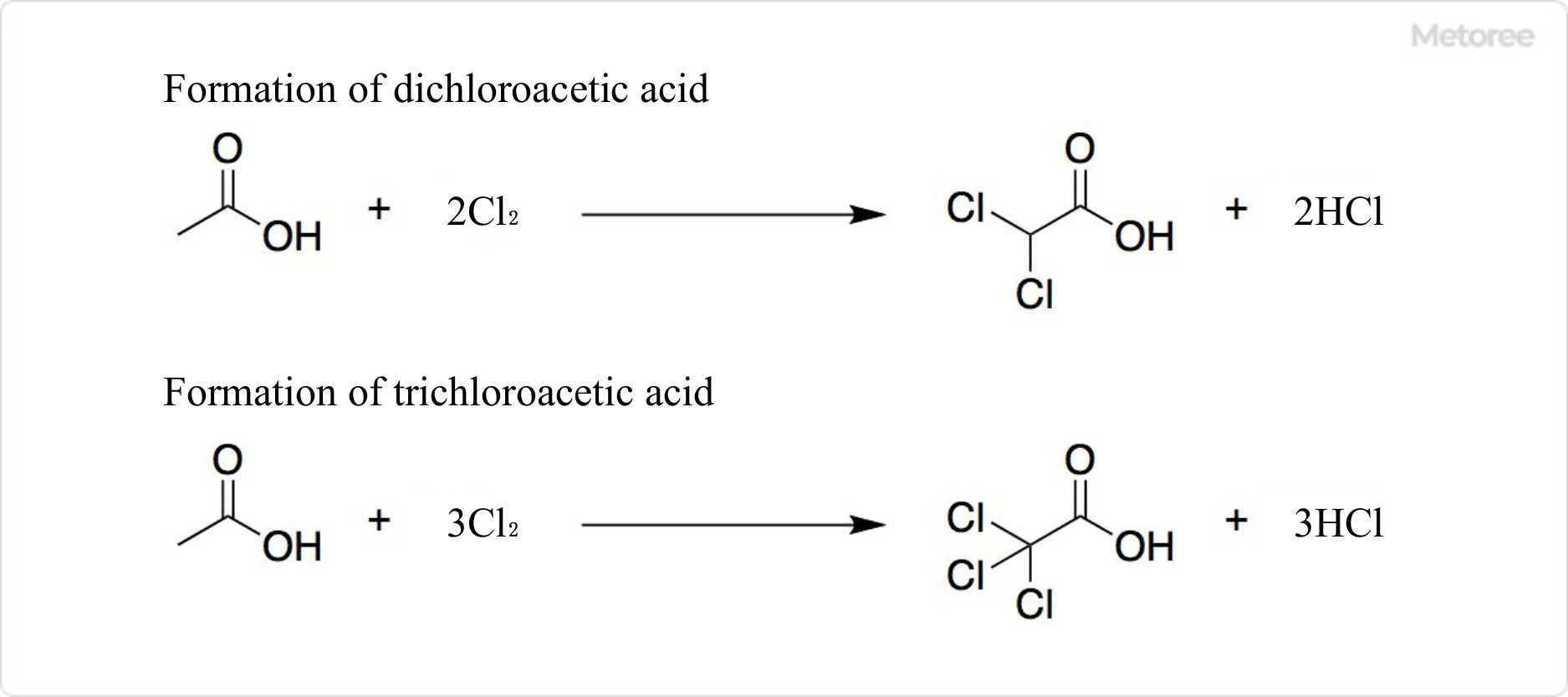
Figure 3. Reaction of chloroacetic acid
Chlorination of acetic acid produces not only chloroacetic acid but also dichloroacetic acid and trichloroacetic acid.
Dichloroacetic acid and trichloroacetic acid are impurities that are difficult to separate by distillation.
In organic chemistry, they are used in the O-alkylation of salicylaldehyde. Decarboxylation of the resulting ether can produce benzofuran.
4. Applications of Chloroacetic Acid
Many reactions with chloroacetic acid will take advantage of the high reactivity of the C-Cl bond. Chloroacetic acid is used in the synthesis of carboxymethylcellulose and carboxymethylstarch, which are thickening agents.
Phenoxy herbicides can be produced by etherification with chlorophenol. For example, 2-methyl-4-chlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid, and 2,4,5-trichlorophenoxyacetic acid can be synthesized.
It is also a precursor to the herbicides glyphosate and dimethoate. chloroacetic acid can be converted to chloroacetyl chloride, a precursor of adrenaline. When chloride is replaced by sulfide, thioglycolic acid can be synthesized, which is used as a stabilizer in polyvinyl chloride (PVC) and as an ingredient in cosmetics.