What Is Tetralin?
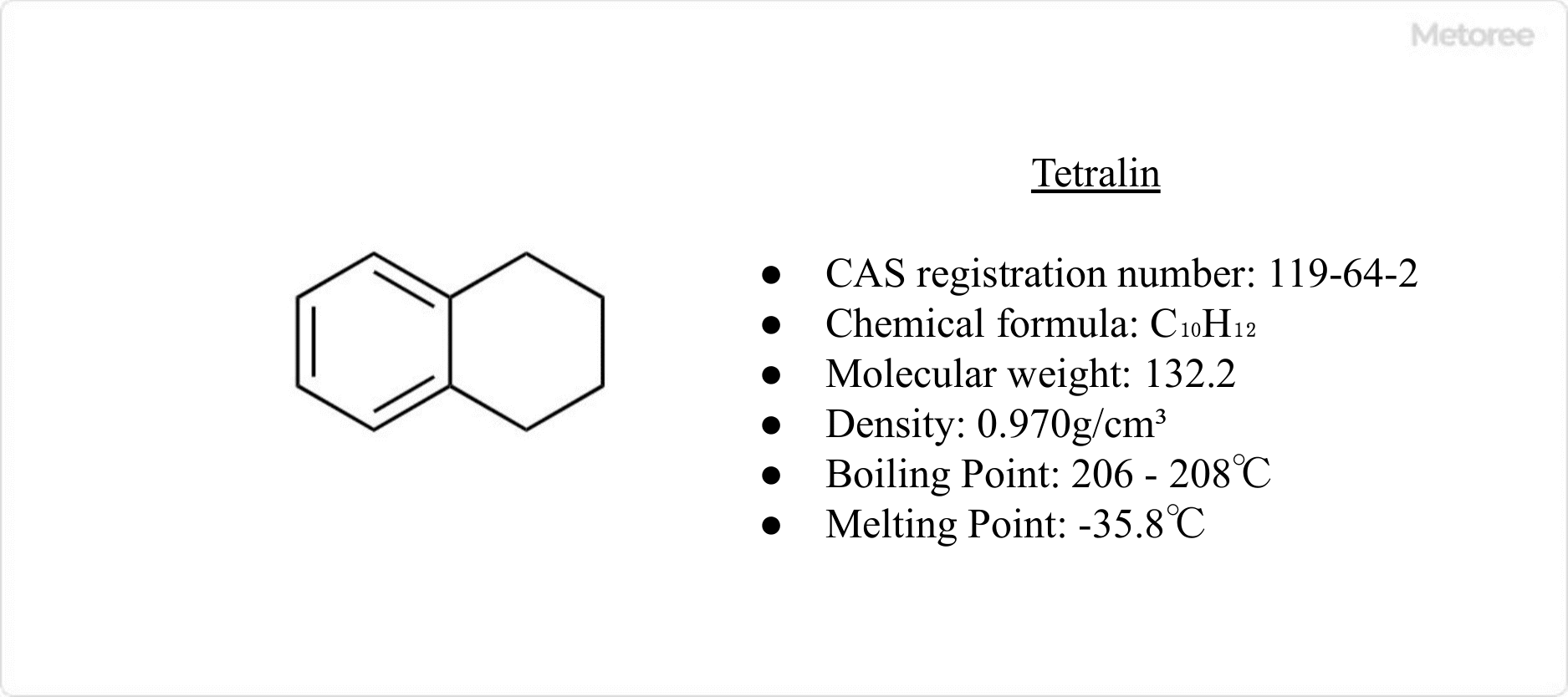
Figure 1. Basic information on tetralin
Tetralin, also known as 1,2,3,4-tetrahydronaphthalene, is an alicyclic compound. It appears as a colorless liquid and is characterized by its hybrid structure, where one benzene ring of naphthalene is hydrogenated and saturated, while the other remains a benzene ring. This gives it both aliphatic and aromatic properties.
Uses of Tetralin
Tetralin is primarily used as a solvent in various applications, including for paints, fats, oils, resins, rubber, waxes, and adhesives. Its high penetrating power makes it effective for cleaning machine parts, particularly in removing oils and fats from intricate details where ordinary solvents are less effective.
It also finds use as a reaction solvent in pharmaceutical intermediate production and decalin synthesis through hydrogenation. Additionally, tetralin reacts with bromine to produce hydrogen bromide, a process utilized in laboratory settings.
Properties of Tetralin
Tetralin is insoluble in water but soluble in organic solvents such as ethanol, ether, and benzene. Its physical properties include a melting point of -35.8°C, a boiling point of 206-208°C, a flash point of 77°C, and an ignition point of 385°C. Oxidation of tetralin leads to the formation of phthalic anhydride, while dehydrogenation results in naphthalene. It is prone to oxidation by air, producing explosive tetralin hydroperoxide, which necessitates caution during distillation, especially in aged samples.
Structure of Tetralin
The chemical formula of tetralin is C10H12, with a molar mass of 132.2 g/mol and a density of 0.970 g/cm3. Structurally, it resembles naphthalene but with one hydrogenated and saturated ring, contributing to its aromatic hydrocarbon nature.
Other Information on Tetralin
1. Synthesis of Tetralin
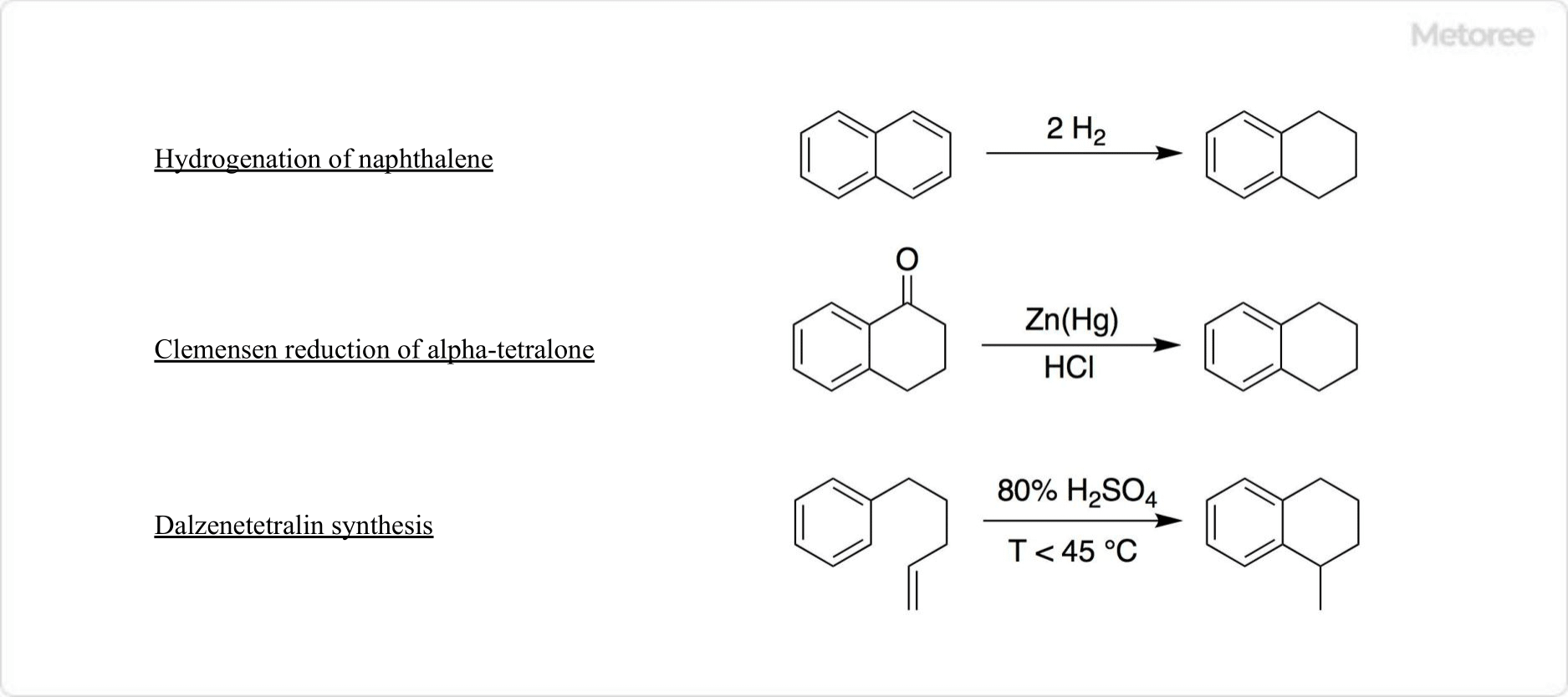
Figure 2. Synthesis of tetralin
Tetralin can be synthesized via nickel-catalyzed hydrogenation of naphthalene or through the Clemmensen reduction of α-tetralone using a zinc amalgam and hydrochloric acid. The classical Darzens tetralin synthesis involves the cyclization of 4-aryl-1-pentene with sulfuric acid to produce methyl tetralin derivatives.
2. Synthesis by Bergmann Cyclization of Tetralin
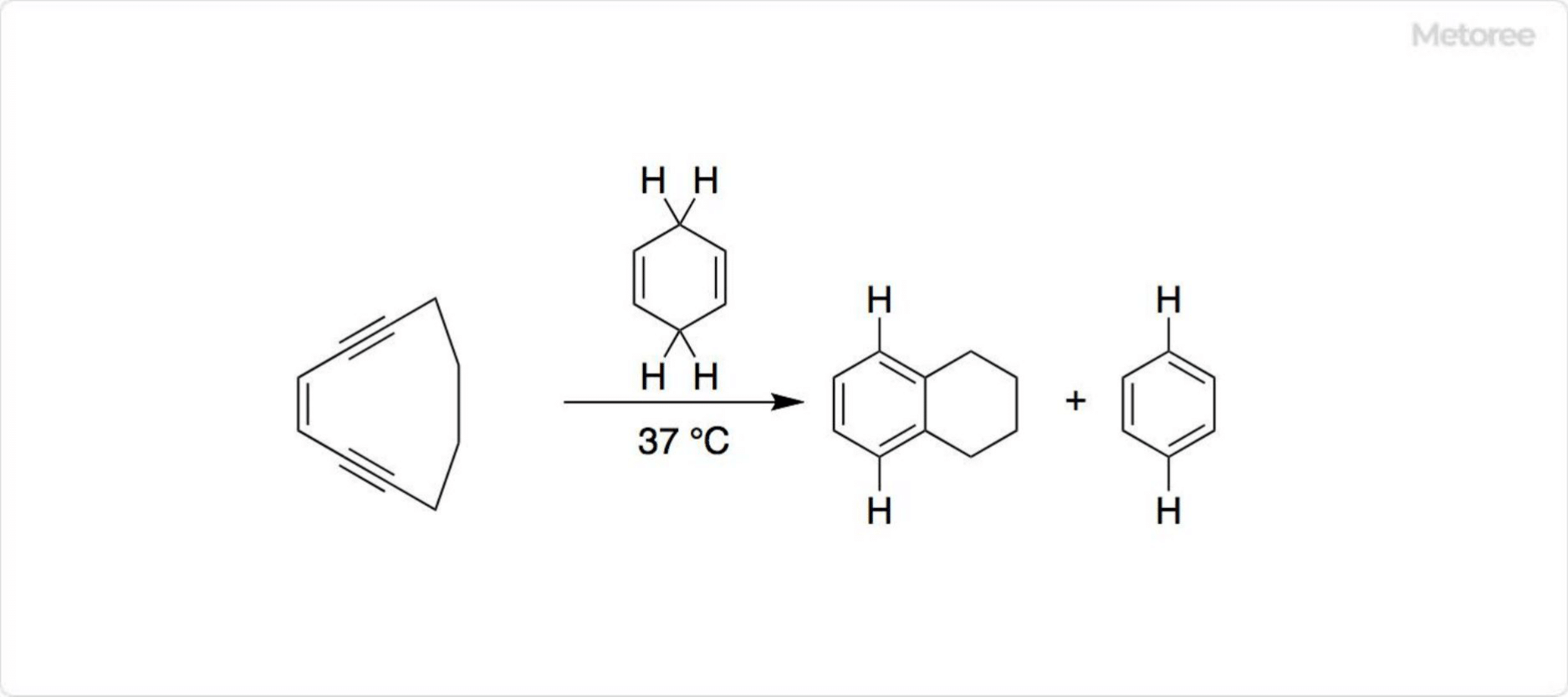
Figure 3. Synthesis of tetralin by Bergmann cyclization
The Masamune-Bergman cyclization, a rearrangement reaction activated by heating enediyne in the presence of a hydrogen donor, is another method for synthesizing the tetralin moiety. The reaction produces a reactive p-benzyne biradical, which can react with various hydrogen donors. For instance, the reaction with carbon tetrachloride yields 1,4-dichlorobenzene, while methanol produces benzyl alcohol. Due to greater ring strain, this cyclization can also occur at lower temperatures, below 37°C, when using larger hydrocarbon rings like cyclodec-3-ene-1,5-diyne.