What Is Oxetane?
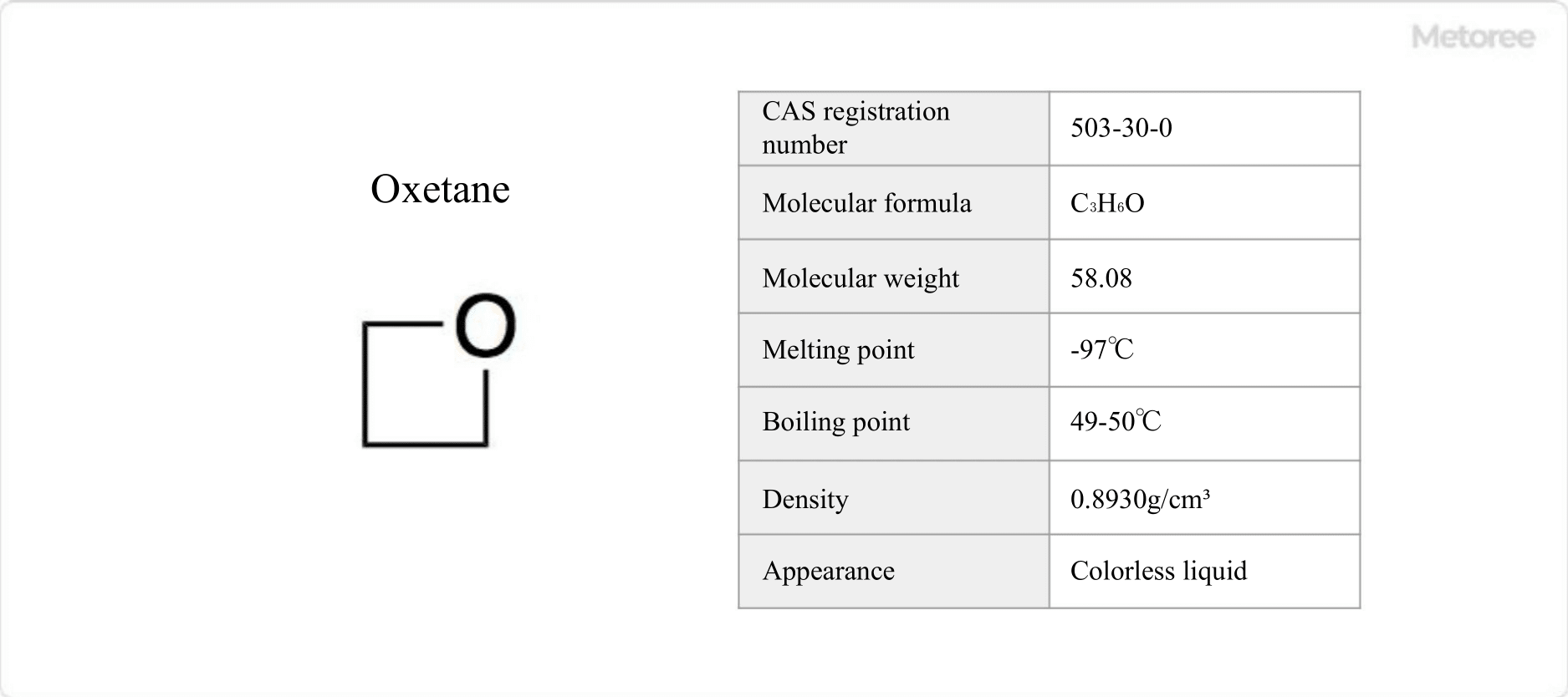
Figure 1. Basic Information on Oxetane
Oxetane is a cyclic ether with a saturated four-membered ring with one oxygen.
It is also known as trimethylene oxide, trimethylene oxide, oxacyclobutane, 3-hydroxy-3-butenyl lactone, 4-methyleneoxetan-2-one, and 1,3-epoxypropane.
In organic chemistry, the four-membered ring structure of oxetane is sometimes referred to as “oxetane” or “oxetane ring.
Uses of Oxetane
Oxetane is used as a raw material for pharmaceuticals, dyes, and preservatives. In organic synthesis, it can be used for hydroxypropylation of amines, alcohols, and benzenes.
Various derivatives containing oxetane are used in additives for epoxy resins, such as paints, adhesives, and various coatings, because of features such as fast curing of epoxy resins and excellent adhesion to plastic materials. In addition to its excellent flexibility and adhesion, it is attracting attention as an environmentally friendly material.
Many compounds with oxetane rings have biochemical characteristics, such as antiviral agents, substances involved in platelet aggregation, and anticancer agents.
Properties of Oxetane
Oxetane has a melting point of -97°C and a boiling point of 49-50°C. It is a colorless liquid with a characteristic odor. It is soluble in organic solvents and miscible with water.
The oxetane ring has high distortion energy and reacts readily with nucleophiles to open the ring. It can be used to hydroxypropylate benzene in the presence of Friedel-Crafts Catalyst as well as amines and alcohols.
Structure of Oxetane
The chemical formula of oxetane is represented by C3H6O or (CH2)3O. Its molecular weight is 58.08 g/mol and its density is 0.8930 g/cm3.
An example of a cyclic ether like oxetane is ethylene oxide (oxirane), a three-membered ring compound with two carbon atoms. Other examples include tetrahydrofuran (THF, tetramethylene oxide), a five-membered ring compound with four carbon atoms, and tetrahydropyran (THP, pentamethylene oxide), a six-membered ring compound with five carbon atoms.
Other Information on Oxetane
1. Synthesis of Oxetane
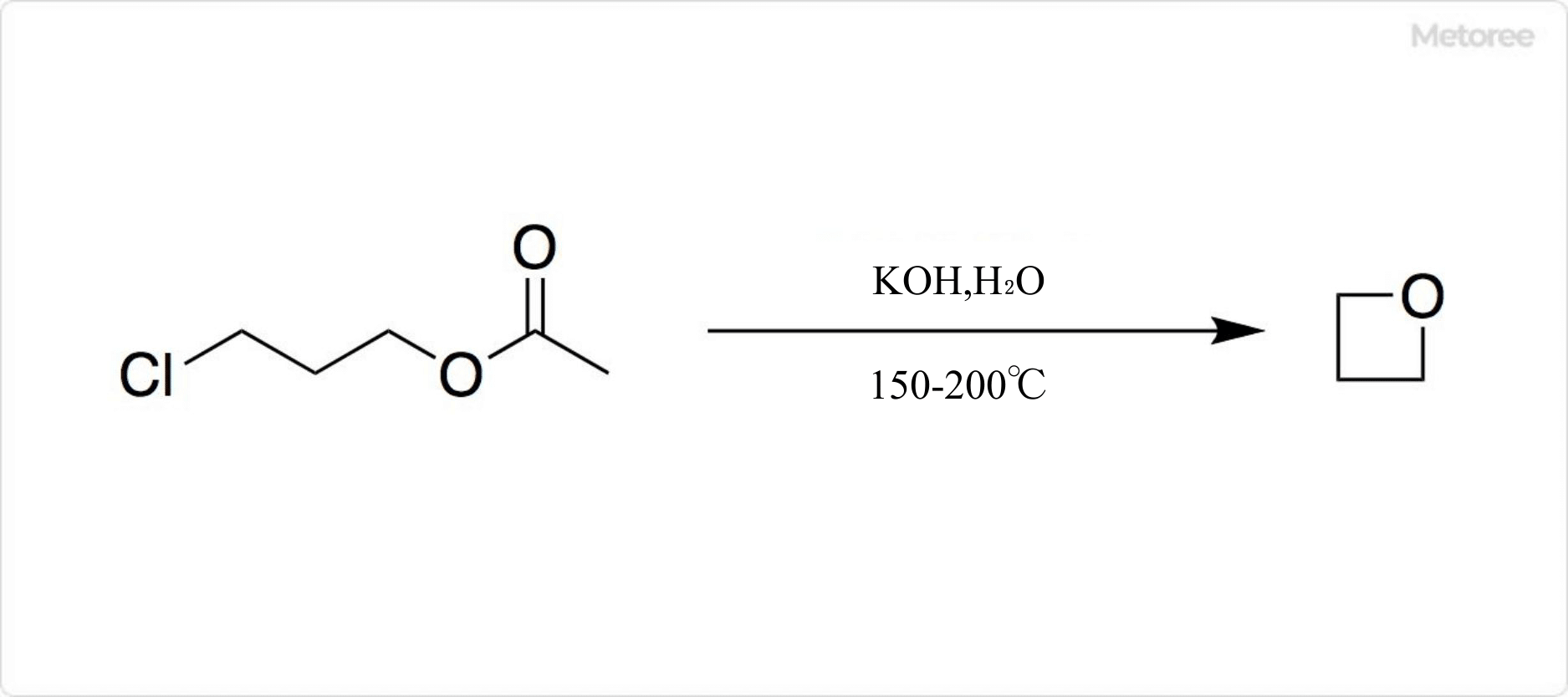
Figure 2. Synthesis of Oxetane
The reaction of potassium hydroxide with 3-chloropropyl acetate at 150°C yields oxetane. The yield is 40% due to the formation of various byproducts.
Oxetane can also be synthesized by the Paternò-Büchi reaction. When a carbonyl compound and an alkene are irradiated with ultraviolet light, [2+2] cyclization proceeds to form an oxetane ring.
Oxetane rings can also be synthesized by cyclization of diols and decarboxylation of six-membered carbonates.
2. Natural Compounds Containing Oxetane Ring
There are not many natural products containing an oxetane ring, but a typical example is the β-amino acid oxetine. Oxetane has a carboxy group and an amino group on the oxetane ring.
Oxetanosine A has two hydroxymethyl groups on the oxetane ring, with adenine substituted. Analogues with thymine or guanine instead of adenine have also been synthesized: oxetanosine T and oxetanosine G.
Other terpenoids with oxetane rings include taxol, clementine, and teucloxide.
3. Related Compounds With Oxetane Rings
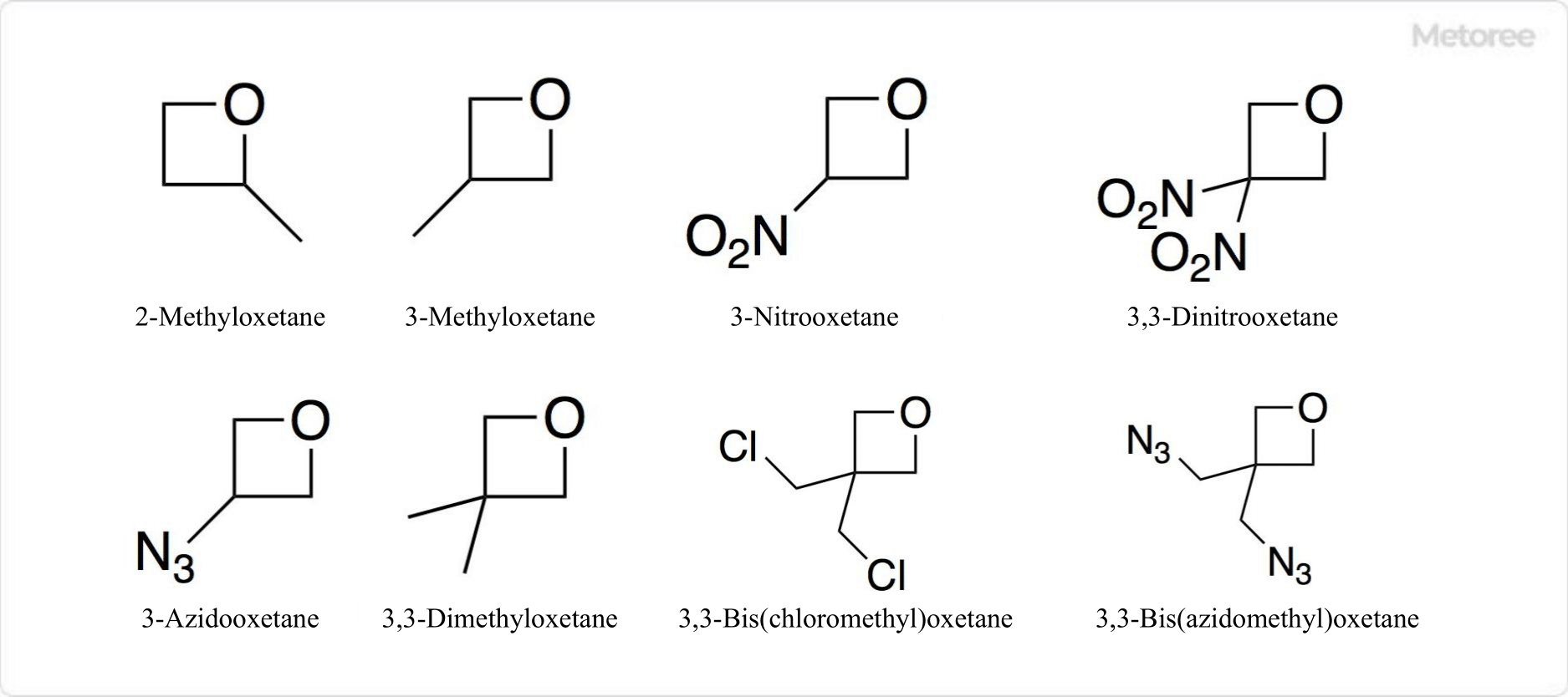
Figure 3. Related Compounds of Oxetane
More than 100 compounds with the oxetane ring have been synthesized. Examples include 3-nitrooxetane and 3,3-dinitrooxetane, which have nitro groups, in addition to 2-methyloxetane and 3-methyloxetane with methyl groups.
Other examples include 3-azido oxetane, 3,3-dimethyloxetane, 3,3-bis(chloromethyl)oxetane, and 3,3-bis(azidomethyl)oxetane.