What Is Erythritol?
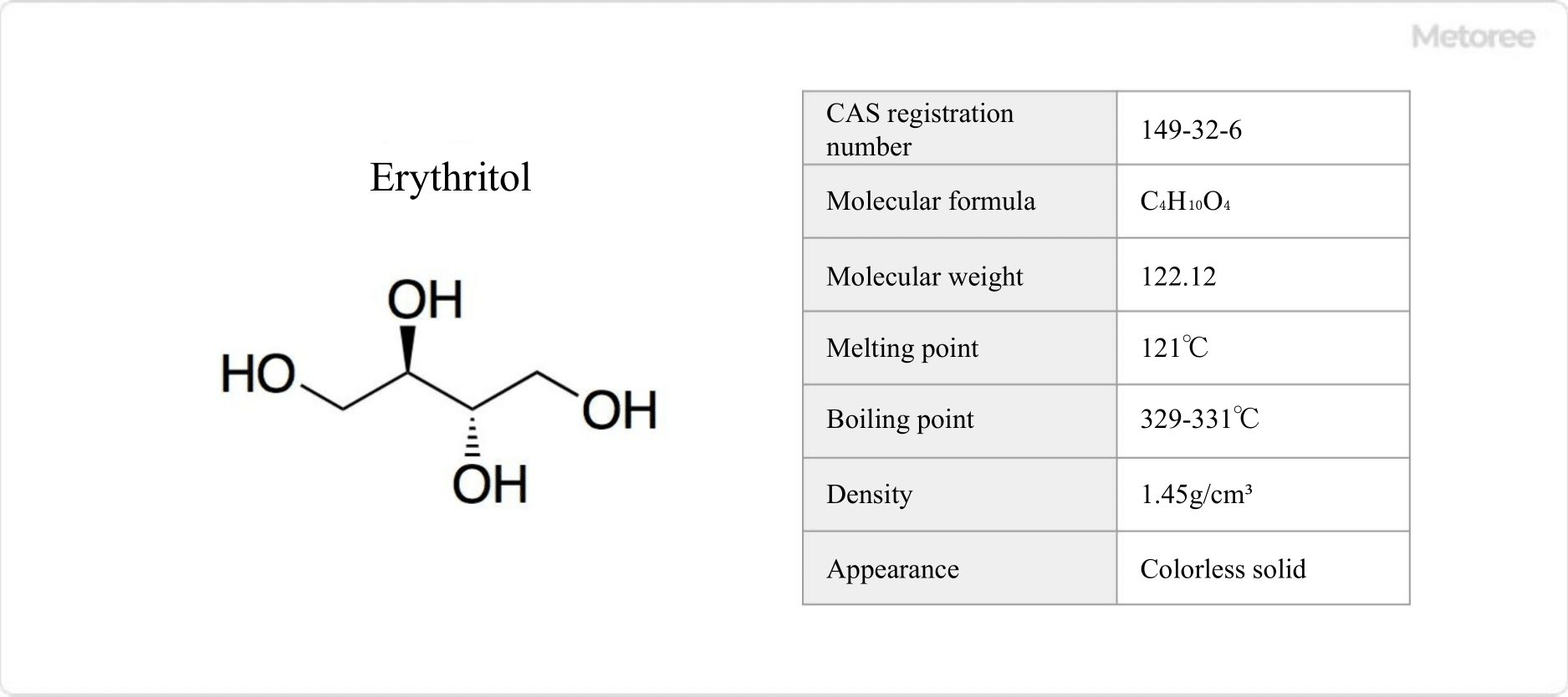
Figure 1. Basic Information on Erythritol
Erythritol is a natural sugar alcohol with the chemical formula C4H10O4.
Examples of natural sugar alcohols other than erythritol include xylitol and sorbitol. Erythritol is found in fruits and mushrooms, as well as in fermented foods such as wine, soy sauce, sake, and miso.
Industrially, it can be produced from corn or wheat starch by fermentation with yeast. Its sweetness level is about 75-80% that of sugar. It is recognized as having zero calories under the Ministry of Health and Welfare’s Energy Evaluation Act.
Uses of Erythritol
Erythritol has almost no calories and is effective in reducing obesity and blood sugar levels when substituted for sugar. Therefore, it is mainly used as an alternative sweetener to sugar.
Examples of common foods containing erythritol include candy, gum, and soft drinks. It can be used as a specific health food to reduce the occurrence of tooth decay or as a sweetener in foods for the sick, such as diabetics.
Furthermore, because of its heat-dissipating effect, erythritol is also used as a cosmetic ingredient in lotions and other moisturizing preparations.
Properties of Erythritol
Erythritol has a melting point of 121°C and a boiling point of 329-331°C. It is a colorless solid. Erythritol absorbs heat by dissolution and has a strong cooling effect.
Erythritol is expressed as HO(CH2)(CHOH)2(CH2)OH. Its molar mass is 122.12 g/mol and its density is 1.45 g/cm3.
Other Information on Erythritol
1. Production of Erythritol
Industrially, erythritol is first obtained from corn by hydrolysis of starch to obtain glucose. The glucose is then fermented by Candida magnoliae, Aureobasidium, Moniliella tomentosa var. pollinis, and other strains of bacteria. Erythritol can be produced by fermentation with
Genetically engineered mutants of Yarrowia lipolytica can produce erythritol by fermentation. Using glycerol as a carbon source, the yield can be increased up to 62% by high osmotic pressure.
2. Isomers of Erythritol
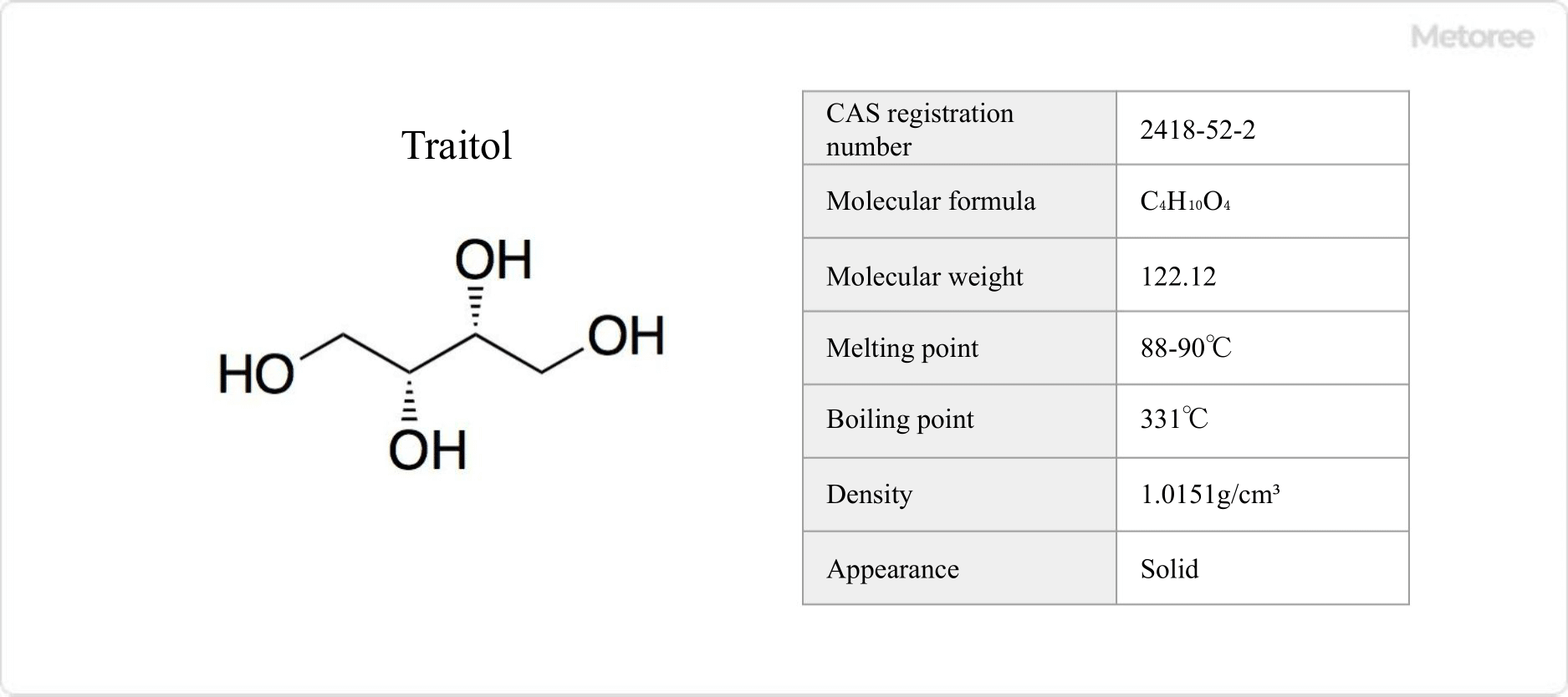
Figure 2. Isomers of Erythritol
Threitol is a diastereomer of erythritol. It is a four-carbon sugar alcohol with the chemical formula C4H10O4. It is mainly used as a synthetic intermediate for various compounds. It has a melting point of 88-90°C, a boiling point of 331°C, and a density of 1.0151 g/cm3.
In vivo, threitol is found in the edible mushroom, Naratake (Armillaria mellea), which has a density of 1.0151 g/cm3. It is also present in Alaskan beetles (Upis ceramboides) and can be used as a cryoprotectant (anti-freeze).
3. Related Compounds of Erythritol
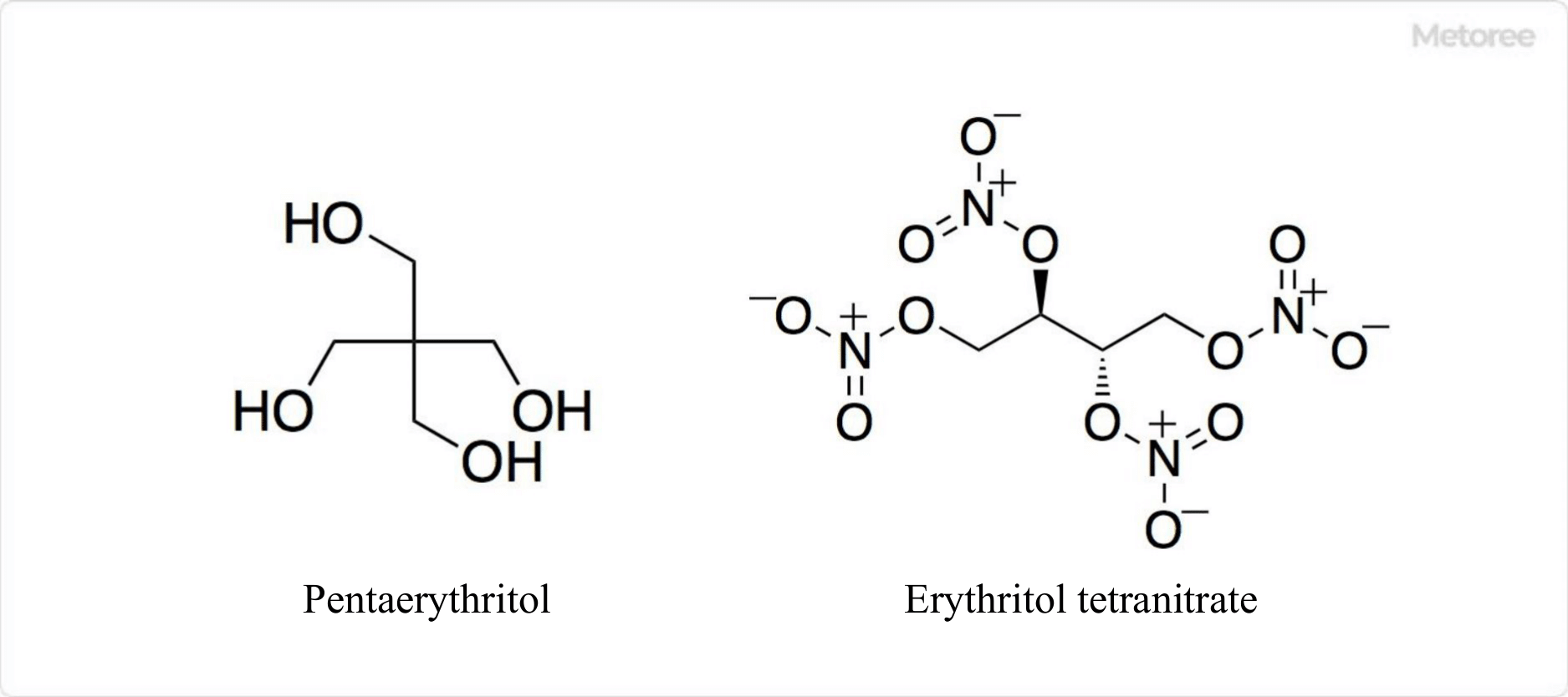
Figure 3. Related Compounds of Erythritol
Pentaerythritol, like erythritol, is a tetravalent alcohol classified as a sugar alcohol. Industrially, it can be used as a raw material for rosin esters, synthetic lubricants, alkyd resins, and explosives.
Erythritol tetranitrate is made by nitration of erythritol with a mixture of concentrated sulfuric acid and nitrate or with a mixture of sulfuric acid and nitric acid. Similar to the high-performance explosive penthrite, it is an explosive compound sensitive to friction and shock. It is used in mixtures with other explosives and should be handled with care as it readily explodes.