What Is Hexenal?
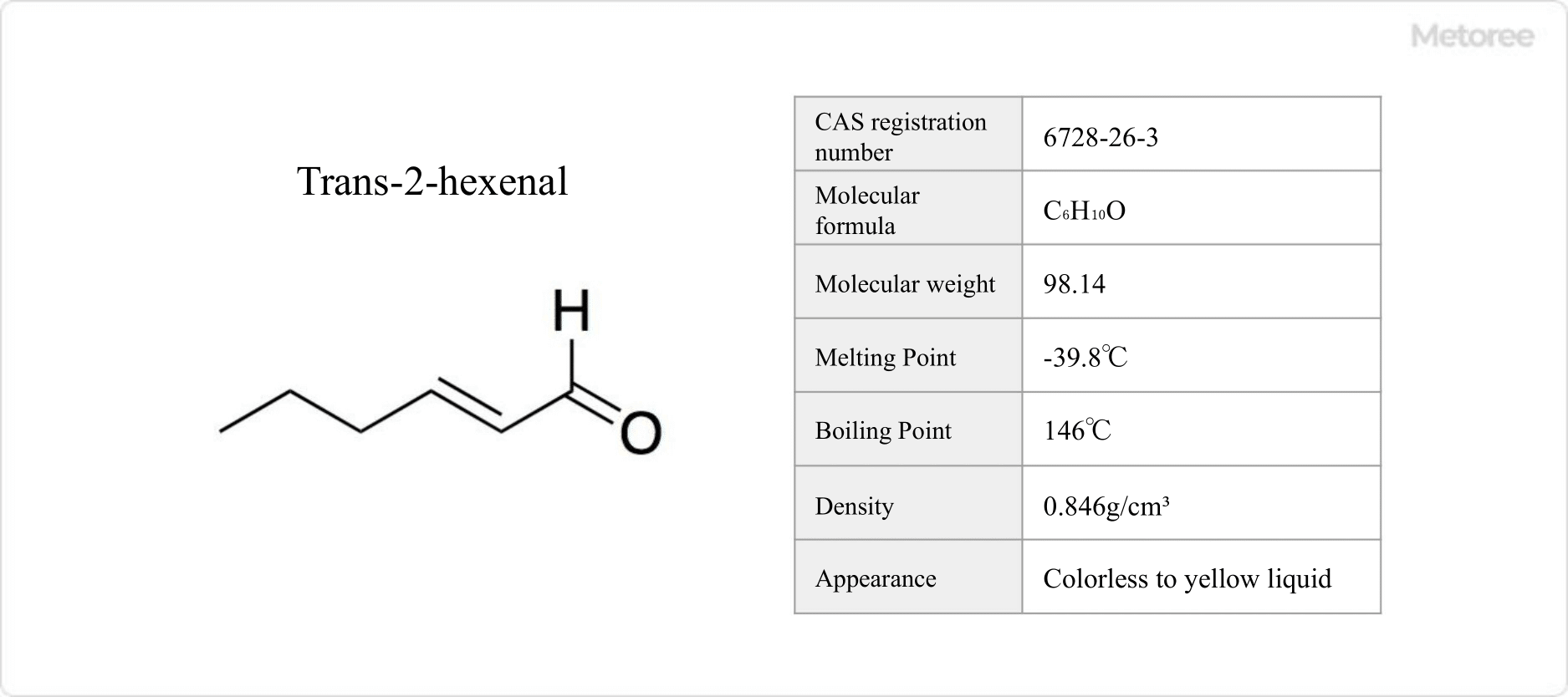
Figure 1. Basic Information on Trans-2-Hexenal
Hexenal, an organic compound with the formula C6H10O, includes isomers like trans-2-hexenal and cis-3-hexenal, also known as Aoba-Aldehydes. It has a molecular weight of 98.14 g/mol.
Uses of Hexenal
The isomers, notably trans-2-hexenal and cis-3-hexenal, contribute to grass and leaf fragrances, serving as flavoring agents. Although both have plant aromas, trans-2-hexenal is preferred for its aromatic quality over the unpleasant odor of cis-3-hexenal.
Properties of Hexenal
Trans-2-hexenal, a colorless to yellow liquid, melts at -39.8°C, boils at 146°C, and has a flash point of 43°C. It dissolves in acetone and ethanol but is nearly insoluble in water. Cis-3-hexenal boils at 126°C.
Structure of Hexenal
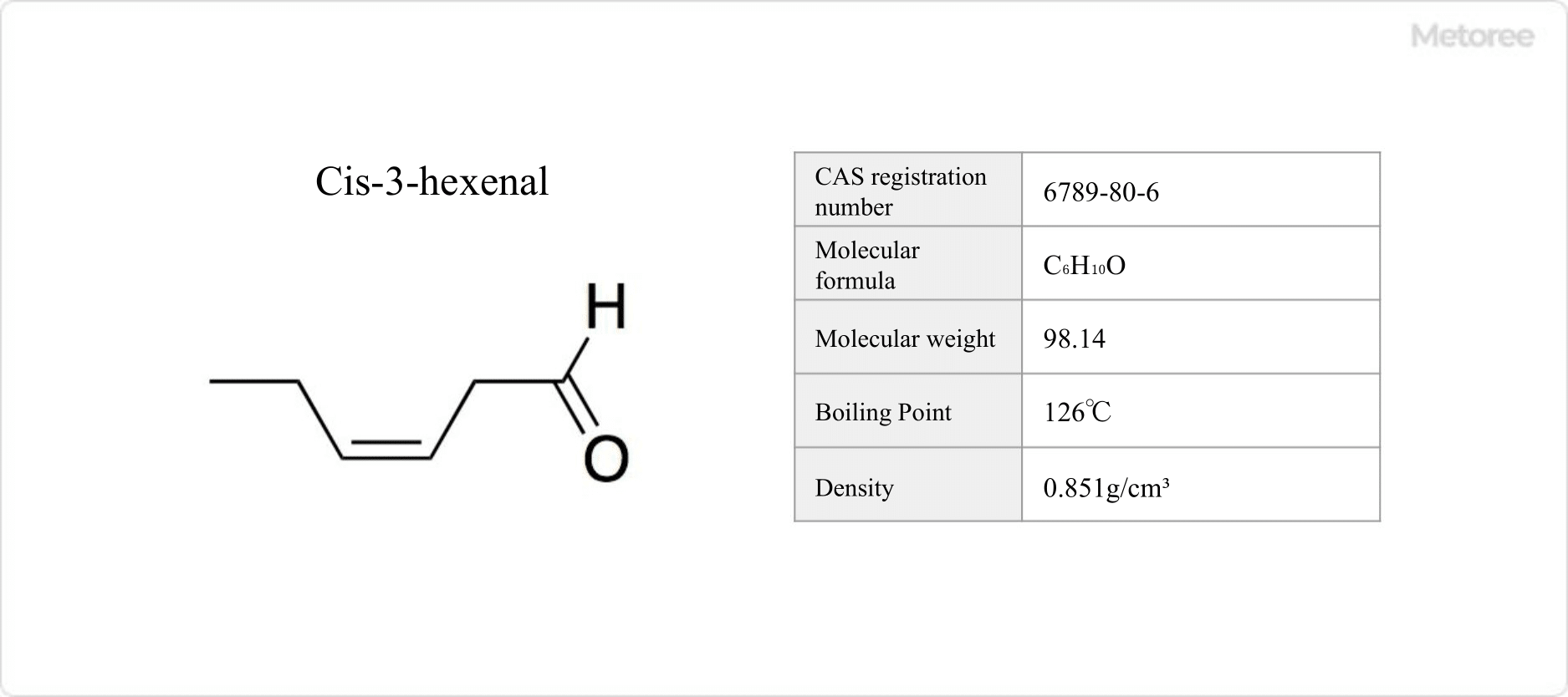
Figure 2. Basic Information on Cis-3-Hexenal
As aliphatic aldehydes, trans-2-hexenal and cis-3-hexenal have densities of 0.846 g/mL and 0.851 g/mL at 25°C, respectively. Cis-3-hexenal can isomerize to trans-2-hexenal, with its alcohol form, cis-3-hexen-1-ol, being a stable and commonly used fragrance.
Other Information on Hexenal
1. History of Hexenal
The identity of tree fragrance during the fresh green season was first explored by Reinke around 1870 through essential oils. Theodor Curtius identified 2-hexenal in 1912, which Akinawa Hatanaka in 1960 confirmed as the same as the Aoba aldehyde from tea.
2. Natural Hexenal
Trans-2-hexenal is found in various vegetables and fruits, making up the stink bug odor. Cis-3-hexenal, prevalent in many plants and used as a pheromone by insects, contributes to the aroma of tomatoes and other produce.
3. Synthetic Method of Hexenal
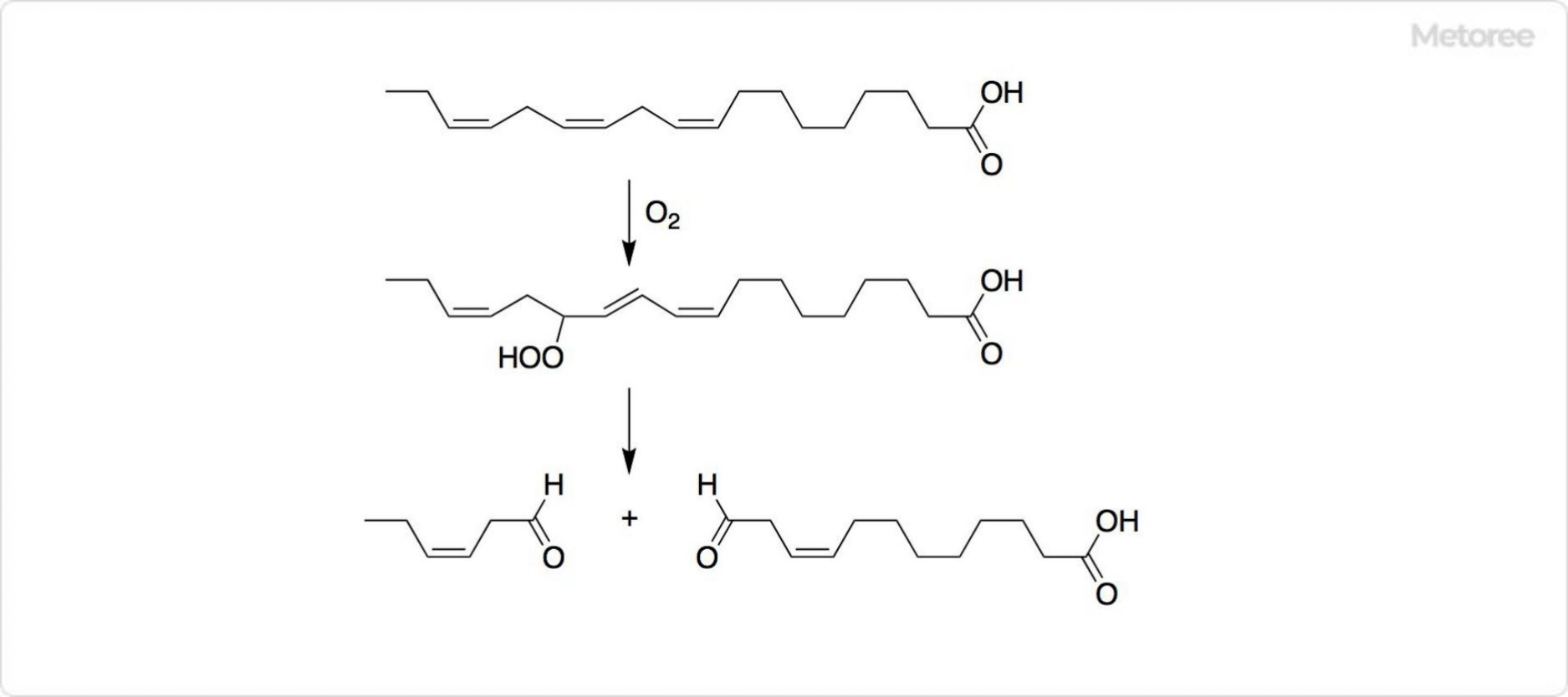
Figure 3. Synthesis of Hexenal
Trans-2-hexenal and cis-3-hexenal are synthesized from ethyl vinyl ether and 3-hexen-1-ol, respectively, with cis-3-hexenal also biosynthesized from linolenic acid.