What Is Phosphinic Acid?
Phosphinic acid is a phosphorus oxyacid with oxidation number I.
Its IUPAC formal name is dihydridodioxophosphoric acid, and its disproportionation reaction occurs above 130 °C to form phosphoric acid (H3PO4) and phosphine (PH3).
It has strong reducing properties and can reduce Cu2+ and Ag+. It is deliquescent and soluble in water, alcohols, and ethers. Its pKa is 1.244 at 25 °C.
Uses of Phosphinic Acid
Phosphinic acid is widely used as a catalyst and reducing agent in organic synthesis, surface treatment agents for metals and other materials, antioxidants, thermal alteration inhibitors, and plating materials. It is also used as raw material for phosphinic acid salts.
Phosphinic acid is used as a reducing agent in electroless nickel plating on steel and plastics. In this process, phosphinic acid salts are oxidized to phosphites, releasing electrons to reduce nickel ions to nickel.
Properties of Phosphinic Acid
Phosphinic acid has a melting point of 26.5 °C. It decomposes at temperatures above 100 °C, forming phosphine (PH3), phosphonic acid (H3PO3), and eventually phosphoric acid (H3PO4).
Structure of Phosphinic Acid
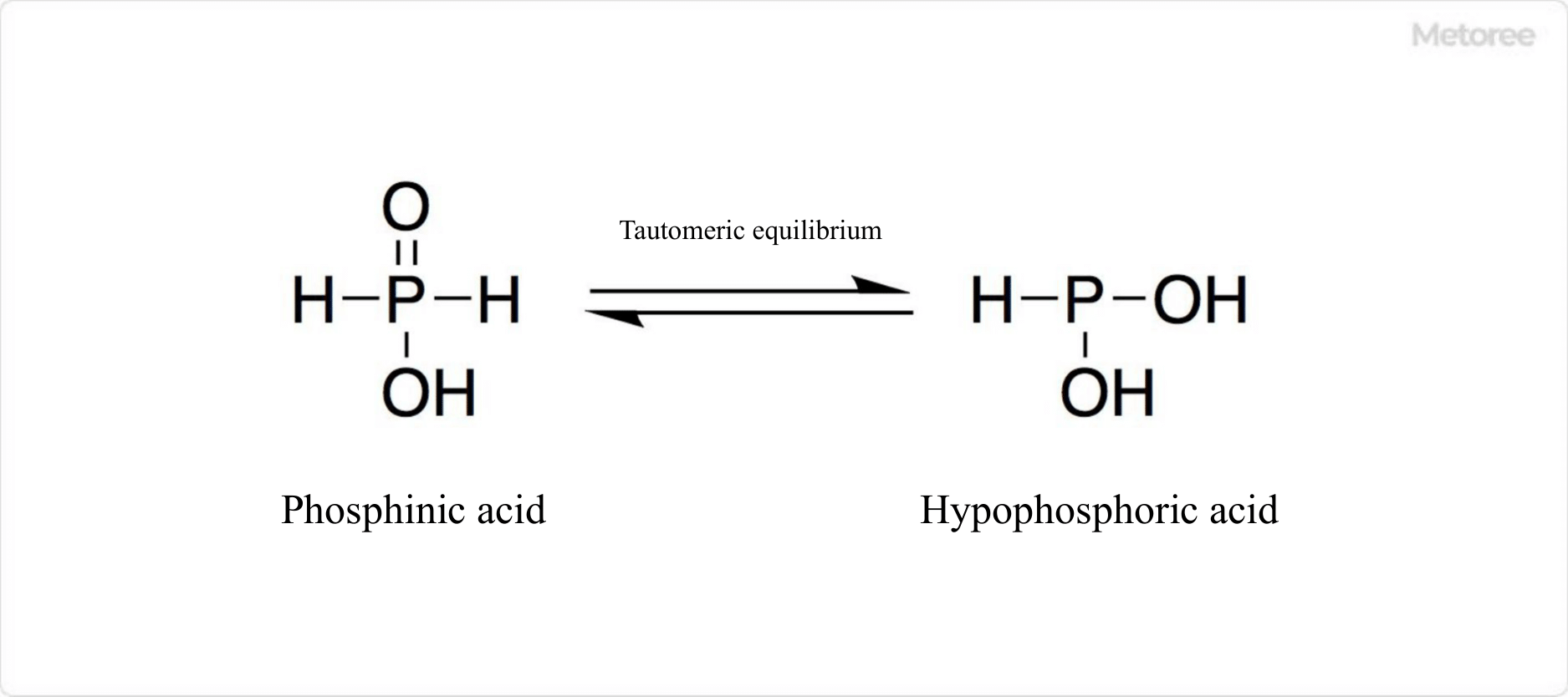
Figure 1. Tautomers of phosphinic acid
Phosphinic acid, with the chemical formula H3PO2, is an inorganic phosphorus compound featuring a P-H bond and a phosphoryl group (P=O). It has a formula weight of 66.0, a specific gravity of 1.45, and a specific formula of (HO)PH2(=O).
The presence of the PH2 group in phosphinic acid has been confirmed by physical measurements such as nuclear magnetic resonance (NMR). It is tautomeric with hypophosphinic acid, which has the same chemical formula.
Other Information on Phosphinic Acid
1. Synthetic Methods for Phosphinic Acid
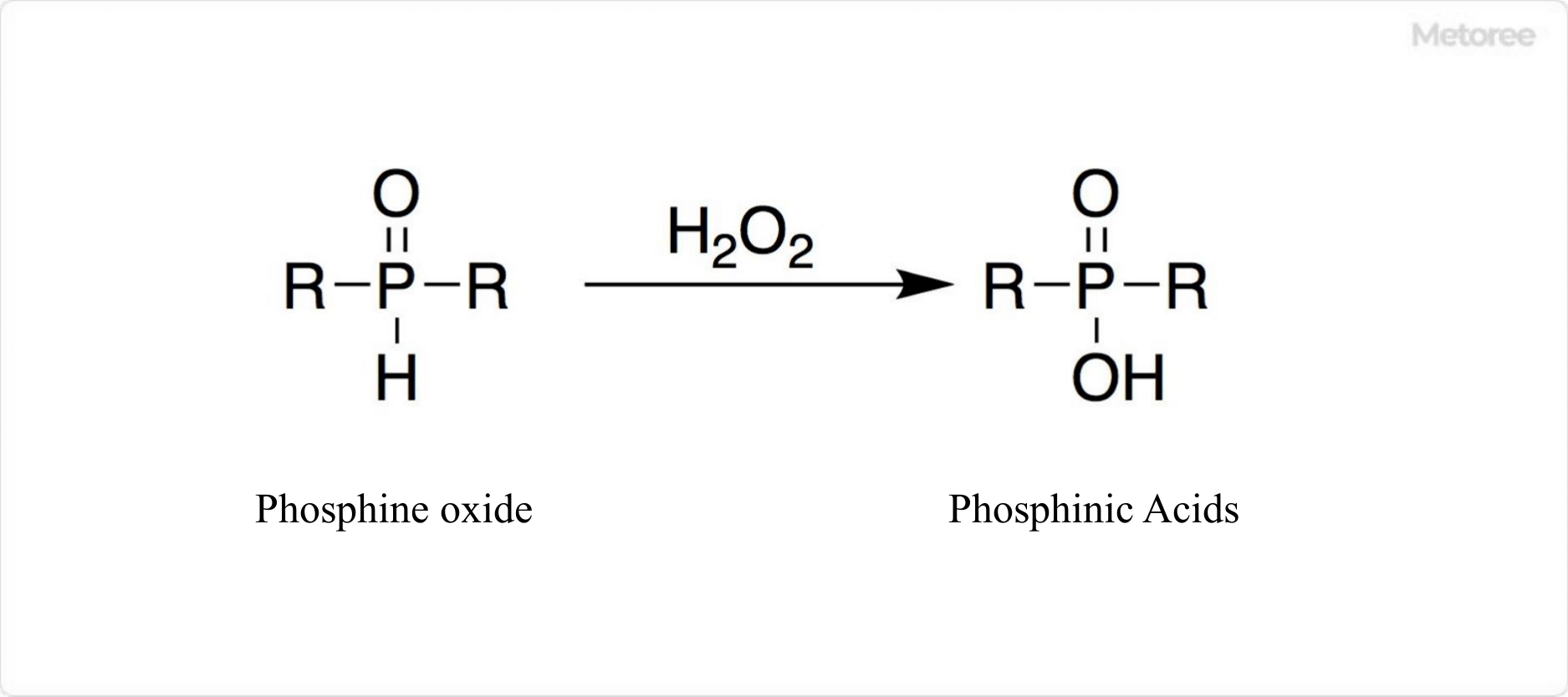
Figure 2. Synthesis of phosphinic acid
To synthesize phosphinic acid industrially, white phosphorus is first treated with slaked lime to produce a calcium salt. This calcium salt is then converted to a sodium salt, resulting in sodium phosphinic acid. Finally, sodium phosphinic acid is converted to phosphinic acid using an ion exchange resin. Alternatively, phosphinic acid can be prepared by oxidizing phosphine oxide with hydrogen peroxide or iodine.
2. Derivatives of Phosphinic Acid
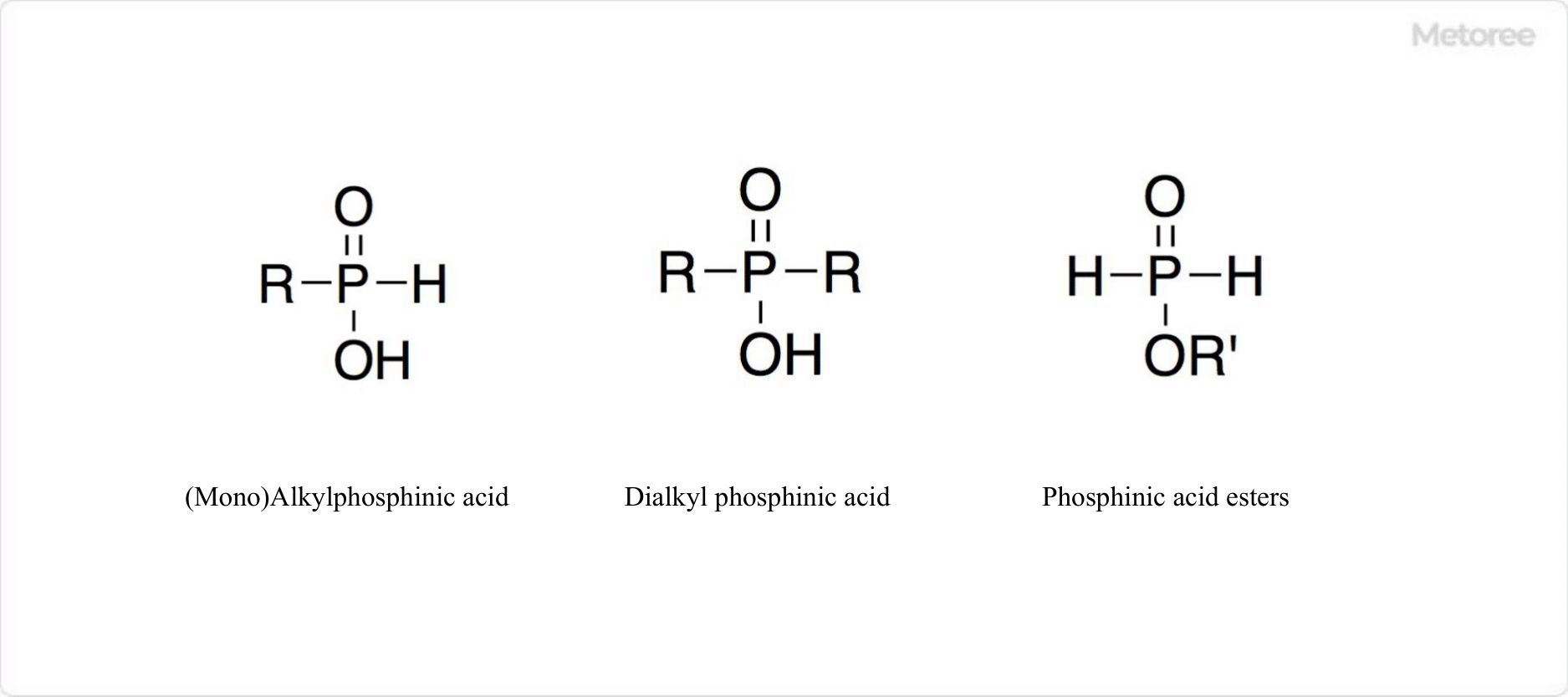
Figure 3. Structure of a derivative of phosphinic acid
Organic derivatives of phosphinic acid include alkyl phosphinic acids. These are compounds where one or two H atoms of the PH2 group are replaced by alkyl group R, forming monoalkyl phosphinic acid (RHP(O)OH) or dialkyl phosphinic acid (R2P(O)OH), respectively. There are also alkyl esters of phosphinic acid, where the H atom of the OH group is replaced by an alkyl group, known as phosphinic acid esters.
3. Related Compounds of Phosphinic Acid
Phosphinic acid is one of several phosphorous oxoacids. Other such oxoacids include phosphoric acid (H3PO4), phosphorous acid (H3PO3), phosphonic acid (H2PHO3), and peroxomonophosphoric acid (H3PO5), with phosphorus oxidation numbers of +5, +3, +3, and +5, respectively.