What Is Acetic Acid?
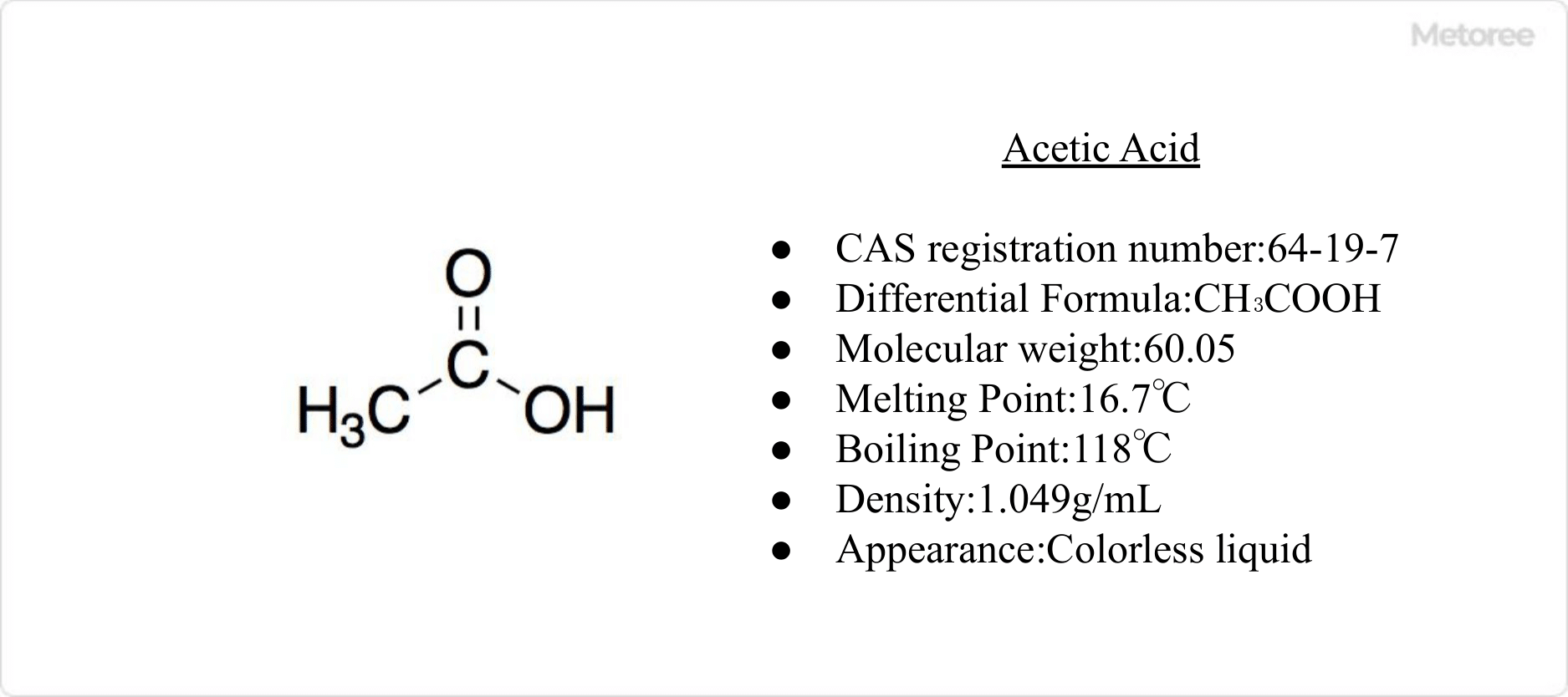
Figure 1. Basic Information on Acetic Acid
Acetic acid, also known as ethanoic acid, is an organic compound that is the main ingredient in vinegar and a type of carboxylic acid. It is characterized as a typical weak acid, presenting as a clear, colorless liquid with a strong sour taste and pungent odor. Produced via chemical synthesis and bacterial fermentation, its synthetic production often involves the carbonylation of methanol, or through the dry distillation of wood.
Acetic acid’s applications span from dyeing and synthetic vinegar to photo-fixing solutions. It is important to note that acetic acid-water mixtures are highly corrosive and require careful handling.
Uses of Acetic Acid
Acetic acid serves primarily as a raw material for chemical synthesis, contributing to the production of vinyl acetate, acetic anhydride, acetone, and various acetic esters and pharmaceuticals. It is instrumental in manufacturing synthetic resins from vinyl acetate and paints from acetic esters, with acetic anhydride acting as a significant acetylation agent for producing acetyl cellulose.
Beyond industrial applications, acetic acid enhances food flavors as a seasoning in vinegar, pickles, and sauces, and is used in flavoring and fragrance manufacturing.
Properties of Acetic Acid
With a melting point of 16.7°C and a boiling point of 118°C, acetic acid is miscible with water, ethanol, ethyl acetate, acetonitrile, chloroform, ether, and benzene, but insoluble in longer-chain hydrocarbons and carbon disulfide. High purity acetic acid, known as glacial acetic acid, crystallizes at low temperatures. Dehydration and condensation of acetic acid molecules result in acetic anhydride.
Structure of Acetic Acid
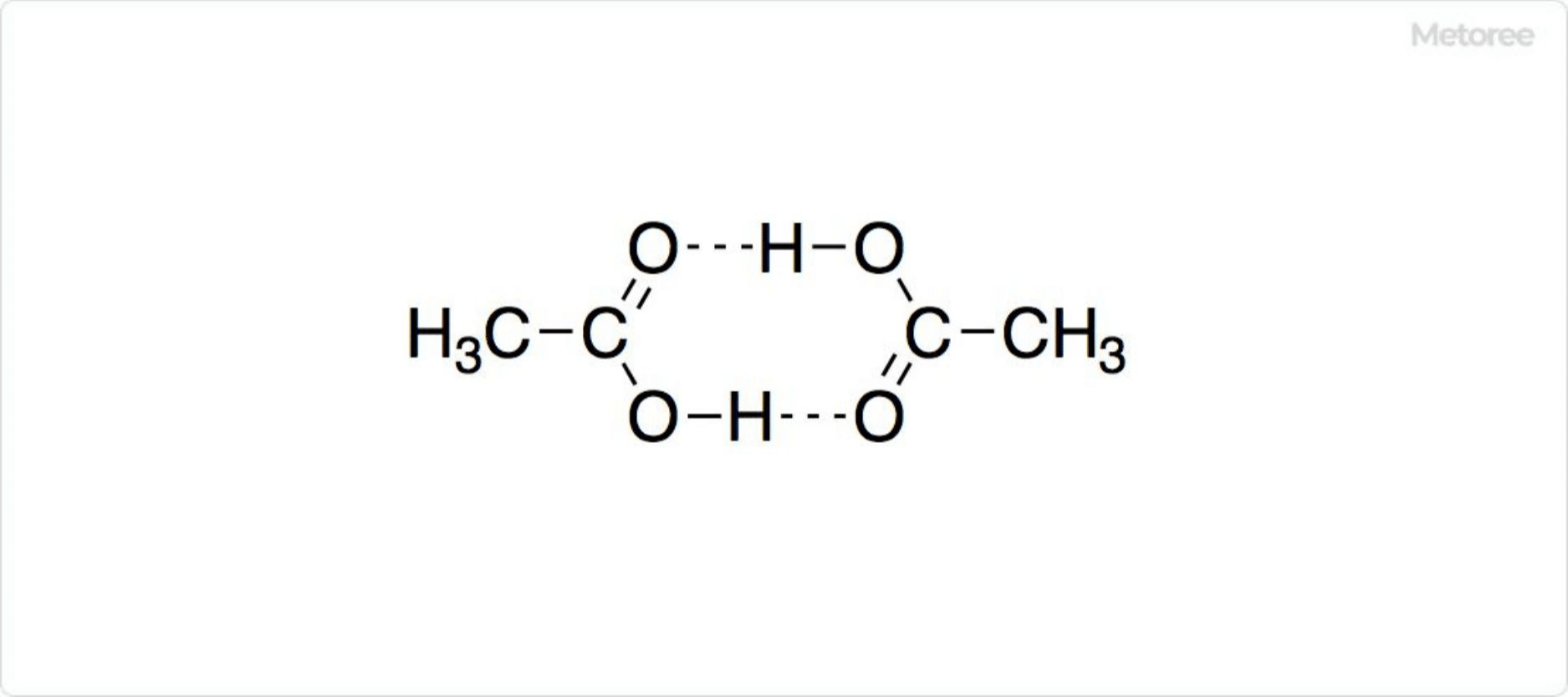
Figure 2. Structure of the Dimer of Acetic Acid
The molecular formula for acetic acid is CH3COOH, featuring carbon and oxygen atoms in the same plane, with bond angles of 119° for C-C-OH and C-C=O, and 122° for O=C-OH. Hydrogen bonding between acetic acid molecules forms a cyclic dimer, observable in gaseous state via electron diffraction and in solid state through X-ray crystallography. In its pure liquid state, acetic acid exists as dimers or polymers, forming dimers in nonprotic solvents and existing as monomers in protic solvents.
Other Information on Acetic Acid
1. Reaction of Acetic Acid
Acetic acid participates in neutralization reactions with bases, producing compounds such as potassium acetate when neutralized with potassium carbonate. It undergoes esterification when heated with alcohols in the presence of sulfuric acid, a process known as Fischer esterification. Additionally, acetic acid can react with chlorine under sunlight to form chloroacetic acid, along with dichloroacetic and trichloroacetic acids as byproducts.
2. Synthesis of Acetic Anhydride
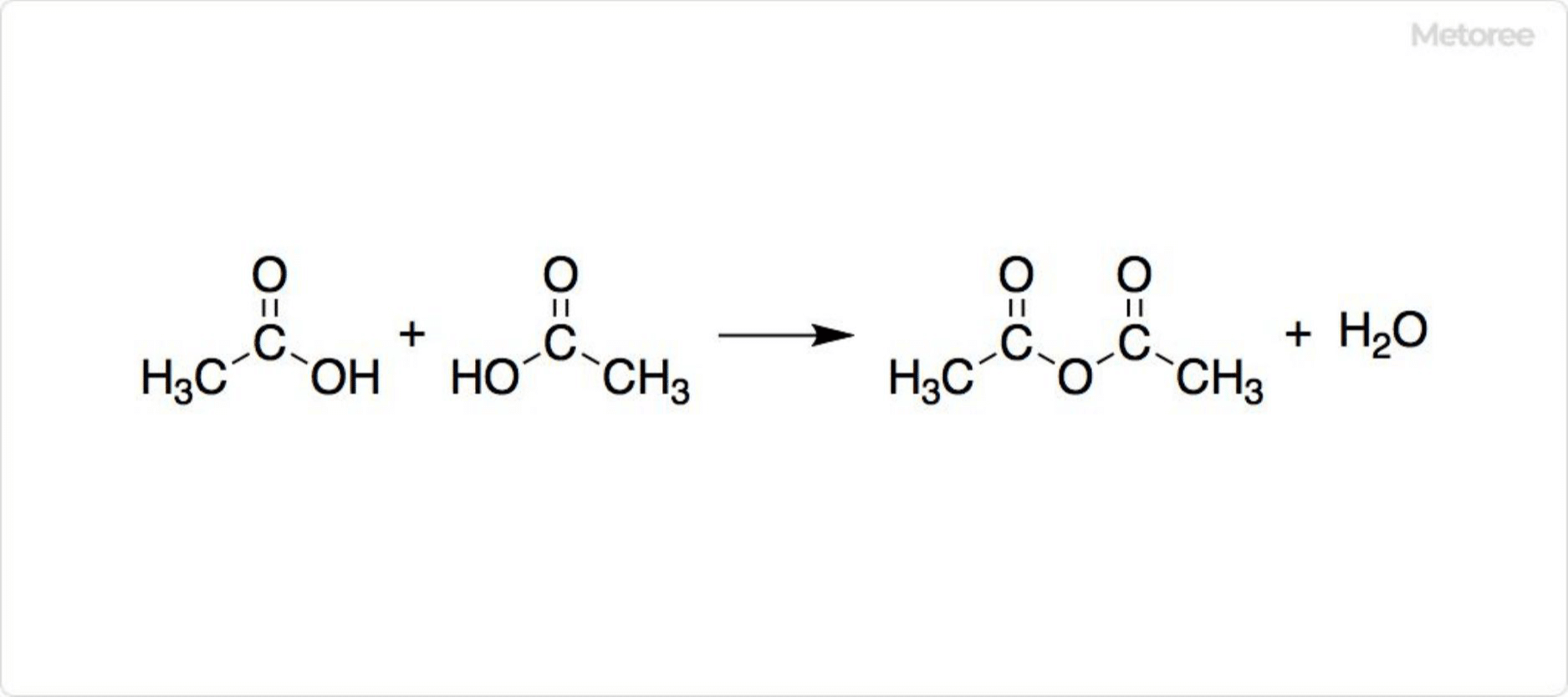
Figure 3. Synthesis of Acetic Anhydride
Acetic anhydride, a carboxylic anhydride, is formed through the dehydration-condensation of acetic acid molecules. It is used as a raw material in the production of cellulose acetate fiber, pharmaceuticals like aspirin, and synthetic materials for fragrances and dyes.