What Is Iodine?
Iodine, a diatomic molecule with the molecular formula I2, is a significant element in the halogen group with atomic number 53. Its CAS registration number is 7553-56-2. Essential for human health, iodine is primarily found in the thyroid gland where it plays a crucial role in hormone production.
Often referred to as the “mineral of the sea,” iodine is plentiful in seaweed and seafood, including kelp and wakame.
Uses of Iodine
Iodine finds extensive applications across various fields, particularly in chemistry and medicine. Key uses include:
- Organic synthesis intermediates and catalysts, stabilizers for organic compounds, rare metals smelting, and analytical reagents
- Pharmaceuticals, dietary supplements, and disinfectants
- Diagnostic and therapeutic radiopharmaceuticals (radioactive iodine-131)
- Livestock feed additives, dyes, photoengraving processes, and agrochemicals
- Thin film thickness measurement, water pipe inspection, and oil field exploration
1. Analytical Chemistry
The addition of starch to an iodine solution triggers an iodo-starch reaction, turning the solution blue-purple. This reaction is a cornerstone of iodometric titration.
2. Medical Field
Iodine is widely utilized as a disinfectant. Common formulations include iodine tincture (an alcohol solution of iodine), Lugol’s solution (a glycerol solution of iodine and potassium iodide), and povidone-iodine (a complex of iodine and polyvinylpyrrolidone).
It is also a key component in contrast media for X-ray diagnostics and various gargles.
3. Other Industrial Applications
As a catalyst in industry and agriculture, iodine serves multiple purposes, including as a raw material for antifungal agents. It’s also finding uses in advanced technologies such as polarizing films for LCD panels and solar cells.
Properties of Iodine
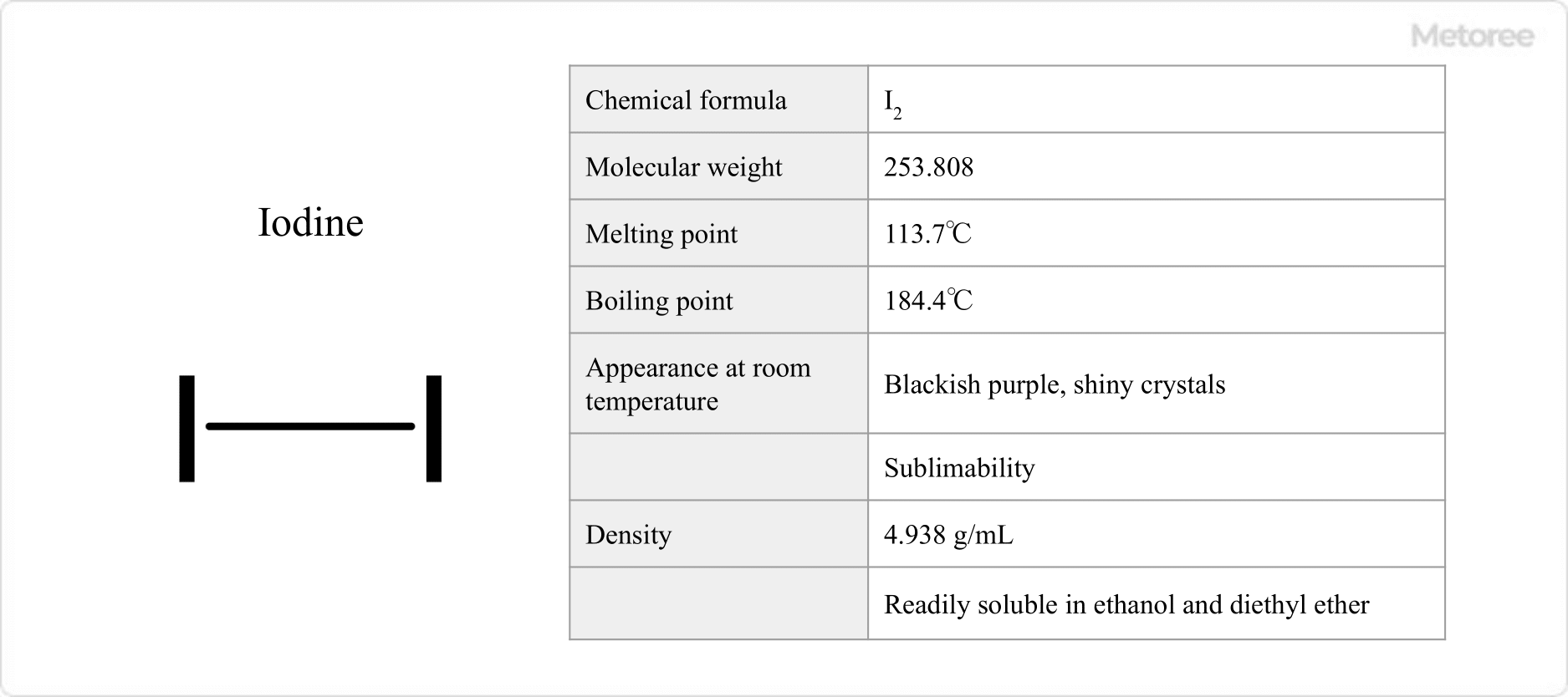
Figure 1. Basic Information on Iodine
Iodine appears as blackish-purple, shiny crystals at room temperature, with a pungent odor and the capability to sublime. It has a molecular weight of 253.808, a melting point of 113.7°C, and a boiling point of 184.4°C. In liquid form, iodine is reddish-brown, and its vapor is purple. The density of iodine is 4.933 g/mL. It is soluble in ethanol and diethyl ether but has limited solubility in water (0.3 g/L).
Types of Iodine
Iodine is available primarily as reagents for research and development and as industrial chemicals.
1. Reagent Products for Research and Development
Used in R&D for pharmaceuticals, reagent preparation, and organic synthesis, iodine is offered in quantities suited for laboratory use, such as 5g, 25g, 100g, and 500g. Due to its sublimation properties, it is typically shipped and stored under refrigeration.
2. Industrial Chemicals
For industrial applications, iodine is packaged in 20 kg and 50 kg fiber drums, facilitating easy handling in factory settings. It is intended for use in a variety of industries, including X-ray contrast media, fungicides, liquid crystals, and more.
Other Information on Iodine
1. Dissolution in Aqueous Potassium Iodide Solution
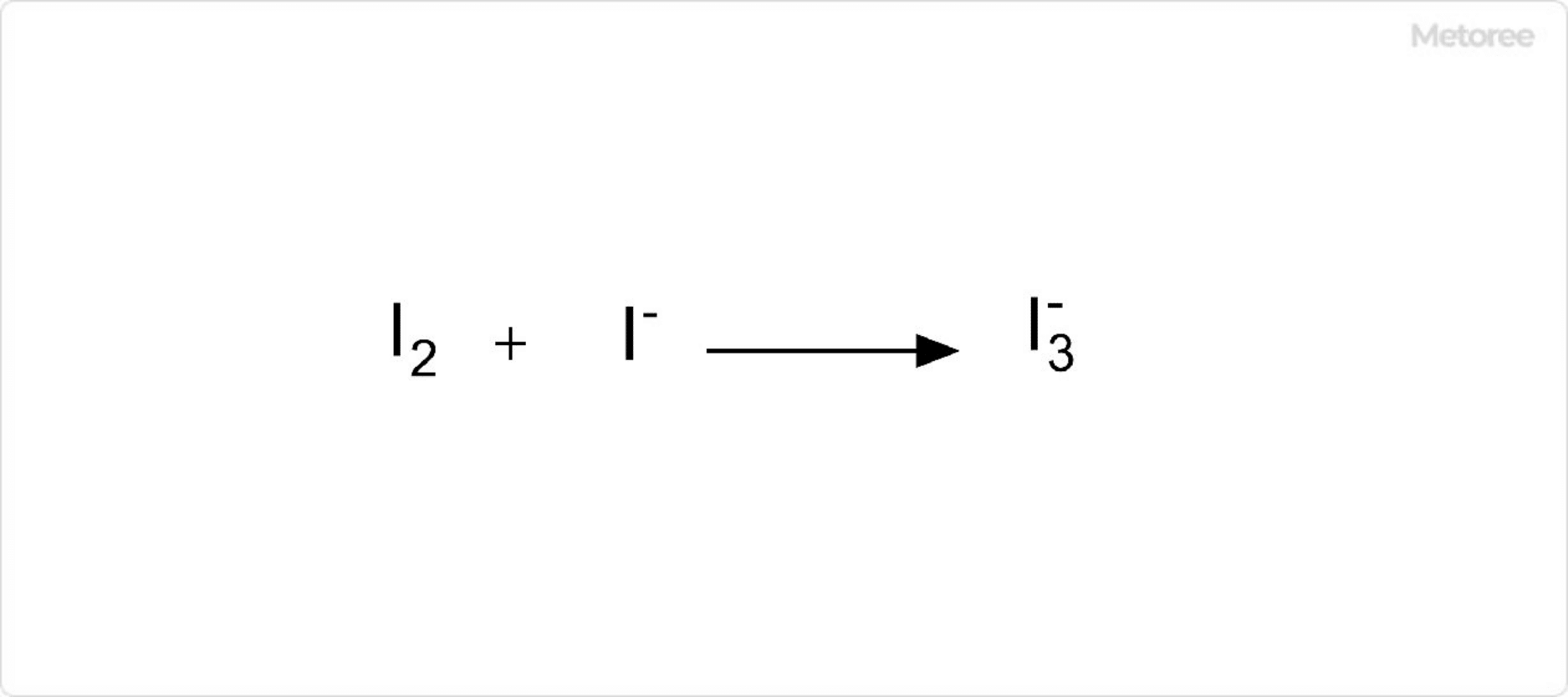
Figure 2. Reaction of Iodine With Iodide Ions
Although iodine has limited solubility in water, it dissolves well in aqueous potassium iodide (KI) solutions, forming triiodide ions through a reaction with iodide ions.
2. Characteristics of Iodine Crystals
Iodine crystals exhibit an orthorhombic structure and are non-magnetic. They have an electrical resistivity of 1.3×107Ω⋅m at 0°C and a thermal conductivity of 0.449 W/(m⋅K) at 300K. Iodine’s oxidizing power is lower than that of other halogens such as fluorine, chlorine, and bromine.
3. Toxicity of Iodine
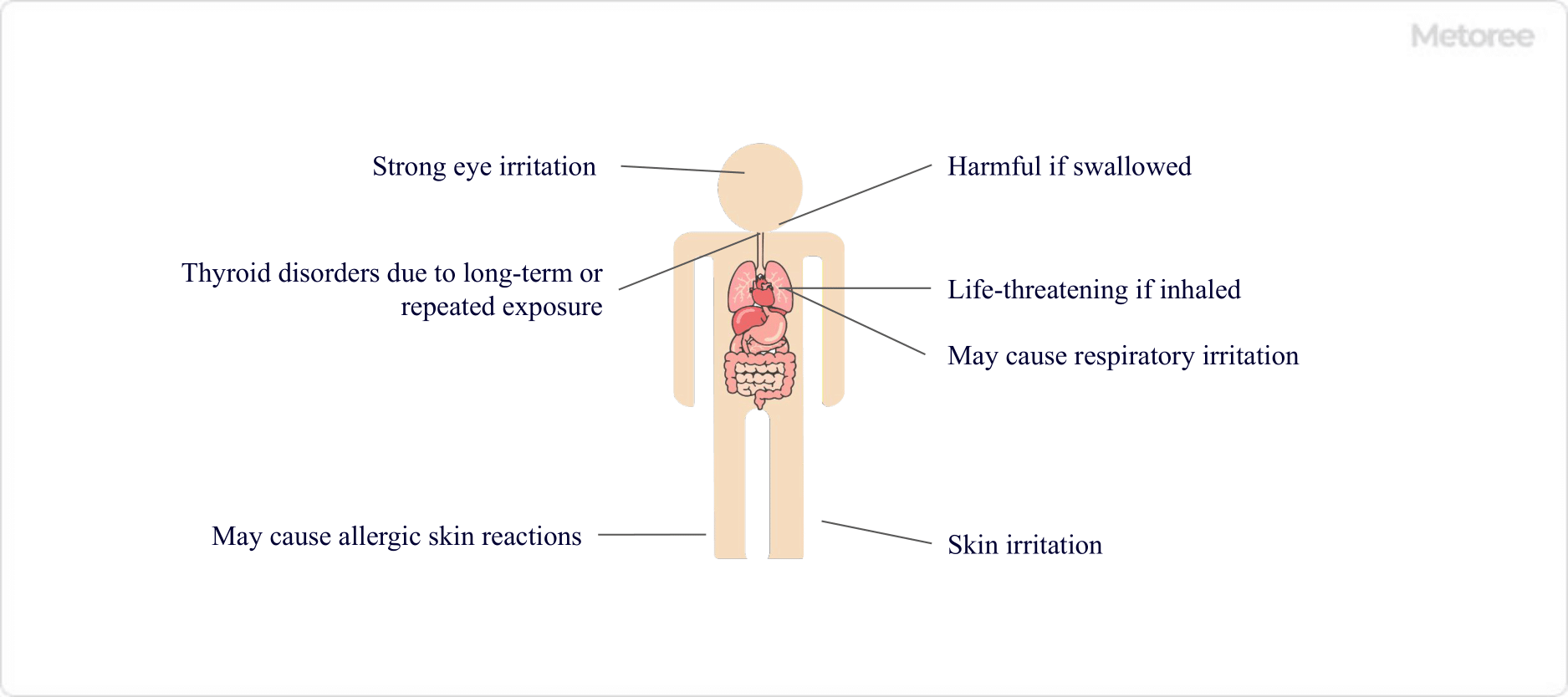
Figure 3. Hazardous Properties of Iodine
Due to its toxic nature, iodine handling requires proper ventilation and protective equipment. It is classified by GHS as follows:
- Acute toxicity (oral): Category 4
- Acute toxicity (inhalation: vapor): Category 1
- Skin corrosion/irritation: Category 2
- Eye damage/irritation: Category 2
- Skin sensitization: Category 1
- Specific target organ toxicity (single exposure): Category 3 (respiratory irritation)
- Specific target organ toxicity (repeated exposure): Category 1 (thyroid gland)