What Is Sodium Hypochlorite?
Sodium hypochlorite is a white solid with the chemical formula NaClO, produced by the reaction of sodium hydroxide solution and chlorine gas. It is typically handled as an aqueous solution due to its instability when exposed to heat, light, and other stimuli. Sodium hydroxide is added as a stabilizer, and the pH is usually adjusted to 12 or higher, making the solution a clear, greenish-yellow liquid with a chlorine-like odor.
Uses of Sodium Hypochlorite
With its strong oxidizing power, sodium hypochlorite decomposes microorganisms and harmful substances. It is utilized across various sectors, including manufacturing, services, domestic settings, and other facilities.
1. Disinfection
Effective against numerous pathogens like norovirus, influenza, salmonella, and E. coli, sodium hypochlorite is a popular disinfectant. Its concentration can be adjusted by dilution, offering versatility in use. After oxidation, it leaves no toxic residues, making it safe for food industry disinfection and water treatment in pools and public baths.
2. Bleaching and More
As an oxidizing agent, sodium hypochlorite is used for bleaching in cleaning and paper industries and household cleaning products. It’s also used in various domestic and industrial applications, including mold decontamination, wastewater treatment, and odor elimination.
Characteristics of Sodium Hypochlorite
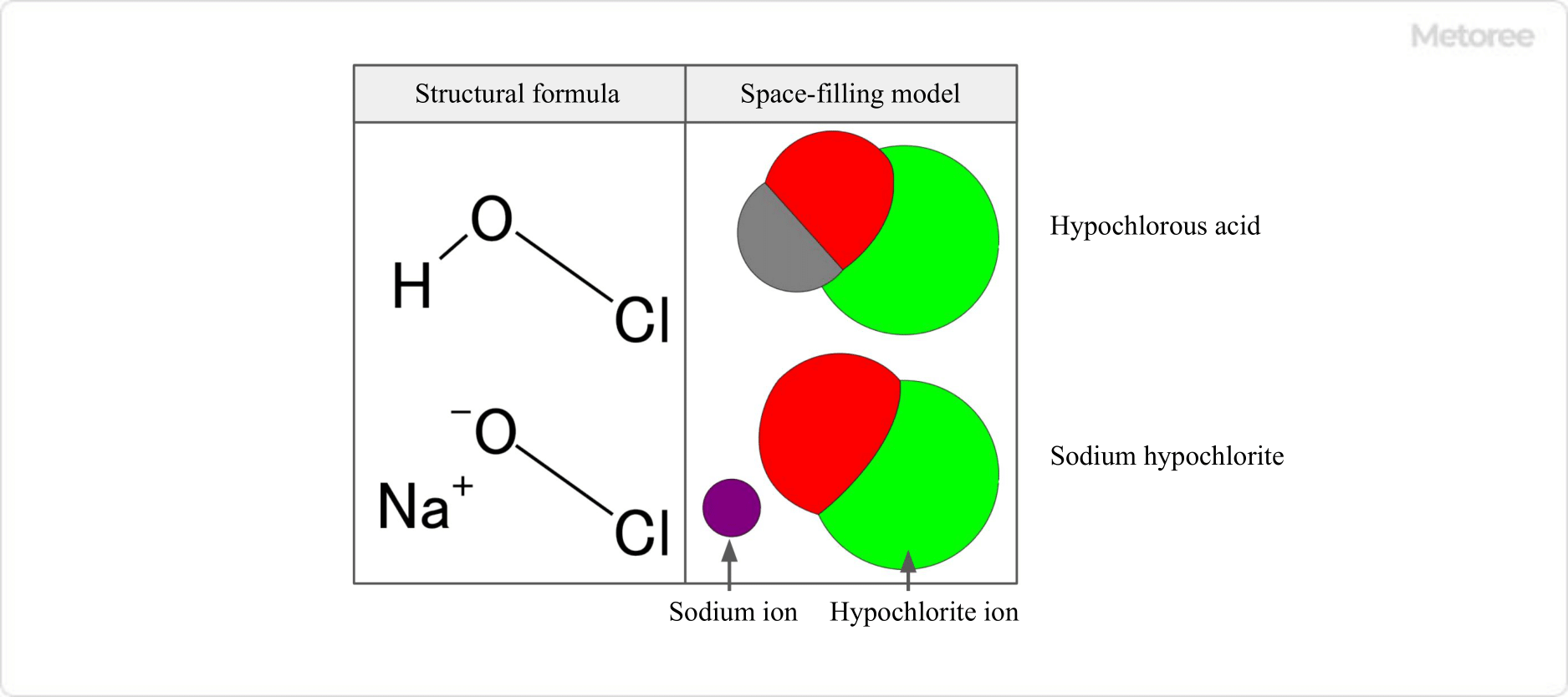
Figure 1. Hypochlorous Acid, Sodium Hypochlorite, and Hypochlorite Ions
Sodium hypochlorite, the sodium salt of hypochlorous acid, has an unstable high concentration and decomposes into sodium chloride and other substances over time. It is usually sold as a strongly alkaline solution with a 4-12% effective chlorine concentration.
Other Information on Sodium Hypochlorite
1. pH Dependence of Hypochlorous Acid Presence
At a pH of 12 or higher, sodium hypochlorite is mostly dissociated into hypochlorite and sodium ions. Dilution with water lowers the pH, increasing the proportion of hypochlorous acid, which has a significantly higher disinfection capability. A pH-adjusted dilution to achieve an effective chlorine concentration of 100 to 500 ppm is recommended for disinfection.
2. Dilution and Usage Concentrations
Follow the product-specific instructions for dilution and use. For self-dilution, use impurity-free water.
3. Effective Chlorine Concentration
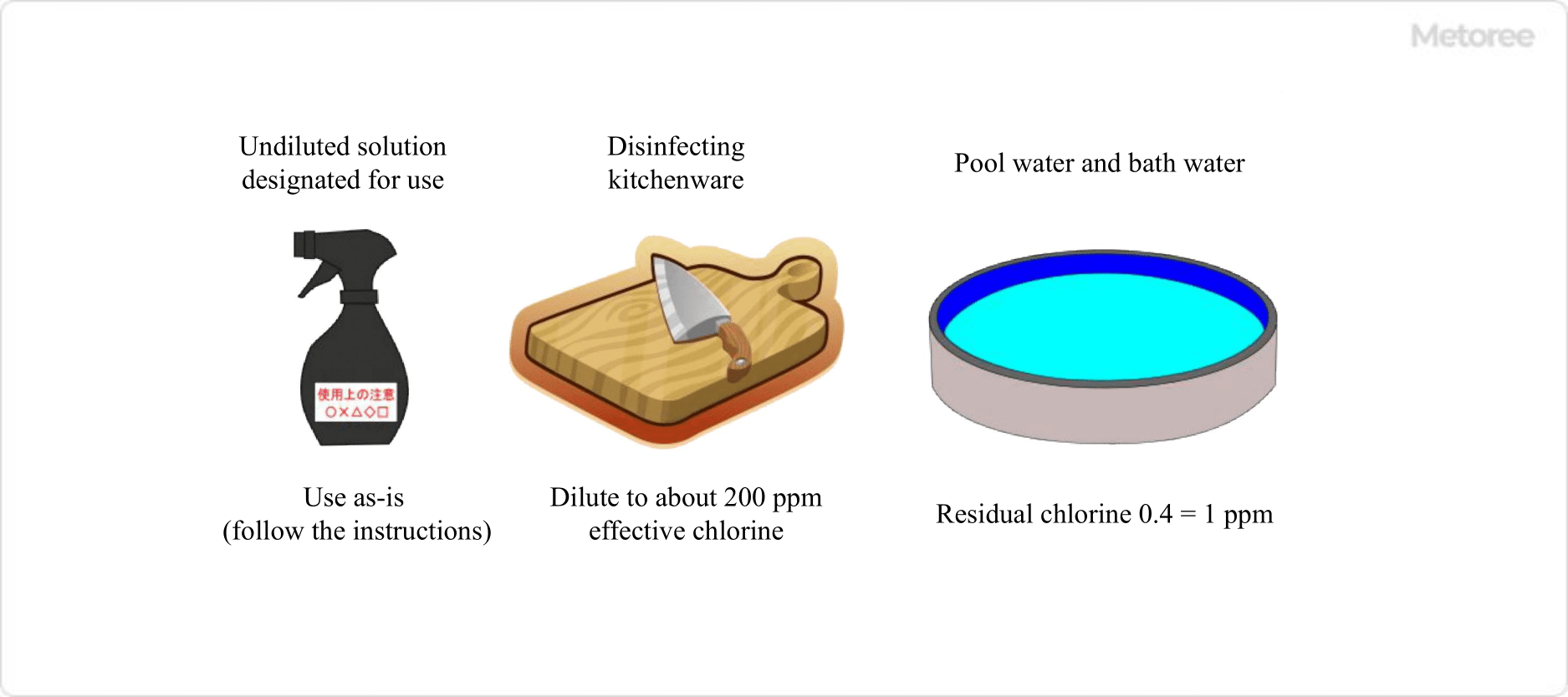
Figure 3. Effective Chlorine Concentration for Various Applications
| Disinfection and bleaching target | Guideline for effective chlorine concentration | Method of use, etc. |
| Raw vegetables and tableware | 100ppm | Soak for 5 to 10 minutes |
| Cutting boards and knives | 200ppm | Wipe clean |
| Sterilization of bathrooms, bathtubs, toilet bowls, etc. | 500ppm | Wipe clean |
| Vomit and other contaminants | 1% | Soak for 5 to 30 minutes |
| Water (drinking water) | 0.1~0.4ppm | Residual chlorine concentration |
| Water (swimming pool, bath water) | 0.4~1ppm | Residual chlorine concentration |
4. Handling Precautions
Sodium hypochlorite solutions, while readily available, are highly alkaline and can cause chemical burns. Protective gear is recommended during use to prevent skin irritation and eye damage. Avoid transferring solutions to unsuitable containers and mixing them with acid detergents, as this can produce toxic chlorine gas.
5. Storage Guidelines
Store sodium hypochlorite in cool, dark, airtight, and non-corrosive containers to prevent decomposition. Ensure it is out of reach of children and regularly check the effective chlorine concentration if stored for more than three months to avoid using ineffective solutions.